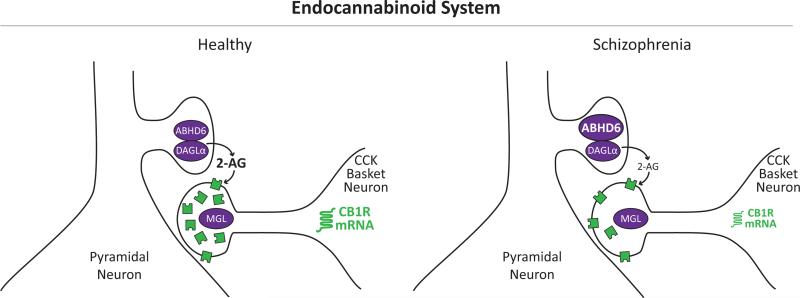
Figure 1. Schematic illustration of the endogenous cannabinoid system in the DLPFC in the healthy state and in schizophrenia.
Left: The synthesizing enzyme for the endogenous cannabinoid 2-AG, diacylglyercol lipase (DAGLα), is localized to the dendritic spines of pyramidal neurons. In response to repeated depolarization, 2-AG is synthesized, travels retrogradely, and activates the endogenous cannabinoid receptor CB1R (green) in nearby inhibitory axon terminals from cholecystokinin (CCK)-containing basket neurons. 2-AG is then degraded by either monoglyceride lipase (MGL) localized to CCK neuron axon terminals or by the serine hydrolase α-β-hydrolase domain 6 (ABHD6) which is co-localized with DAGLα in the dendritic spines of pyramidal neurons. Right: In schizophrenia, higher mRNA levels for ABHD6 (larger font size) have been reported in the DLPFC. Because ABHD6 is co-localized with DAGL in the dendritic spines of DLPFC pyramidal neurons, higher ABHD6 mRNA levels may lead to higher metabolism of 2-AG directly at the source of 2-AG production, which may in turn lower 2-AG activity at CB1R. In addition, studies have found lower levels of CB1R mRNA (smaller font size) and protein immunoreactivity and higher CB1R receptor binding in the DLPFC of the same schizophrenia subjects. One potential explanation for this apparent discrepancy may be the presence of higher levels of membrane-bound CB1R due to altered trafficking that are accessible to ligand binding, while the total amount of intracellular CB1R is lower.