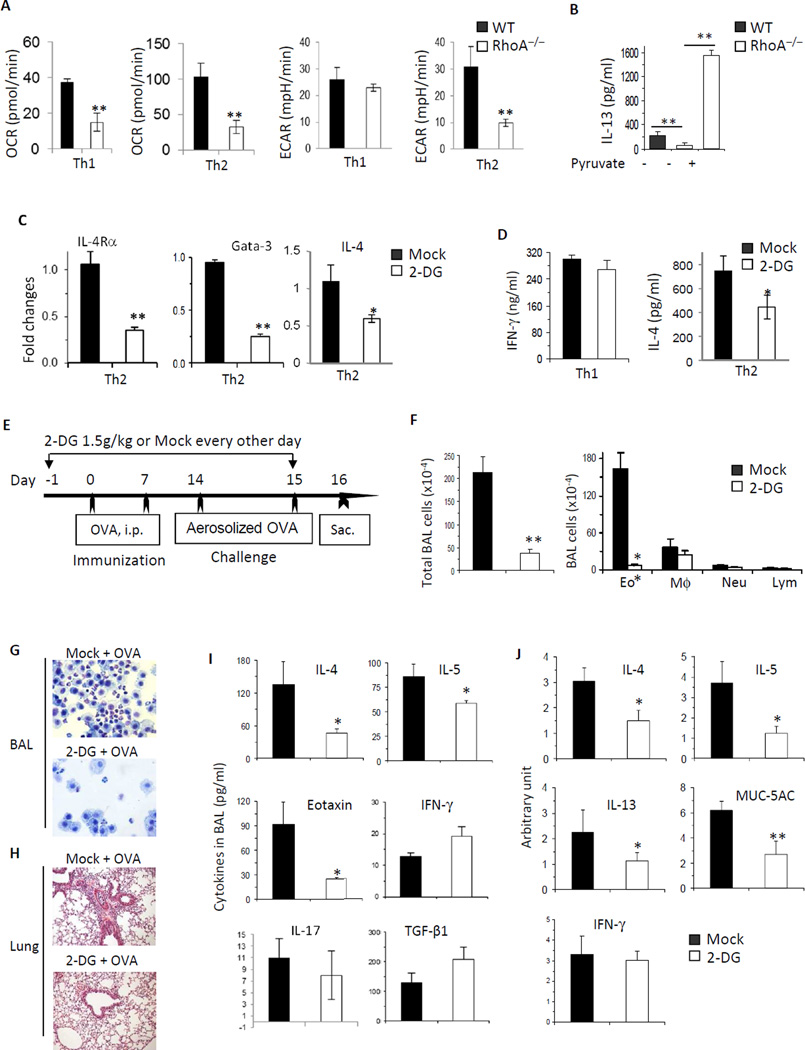
FIG 6. RhoA connects glycolysis to Th2 cell differentiation and allergic airway inflammation.
A, RhoA deficiency impairs OXPHOS in both Th1 and Th2 cells, but glycolysis in Th2 but not Th1 cells. WT and RhoA−/− CD4+ naïve T cells were cultured under Th1- or Th2-skewed conditions for 4 days and restimulated with PMA plus ionomycin for 5 h, followed by measurement of OCR and ECAR. B, Pyruvate rescues RhoA deficiency-induced defect in Th2 cell differentiation. WT and RhoA−/− CD4+ naïve T cells were differentiated under Th2 conditions for 3 days, in the presence or absence of pyruvate (2 mM). IL-13 production was analyzed by ELISA. C, Inhibition of glycolysis impairs IL-4Rα, Gata-3, and IL-4 expression. WT CD4+ naïve T cells were cultured under Th2 conditions in the presence of PBS (Mock) or 2-DG (0.3 mM) for 24 h. mRNA levels of IL-4Rα, Gata-3, and IL-4 were analyzed by real-time PCR. D, Inhibition of glycolysis impairs IL-4 secretion. WT CD4+ naïve T cells were cultured under Th1 or Th2 conditions in the presence of Mock or 2-DG (0.3 mM). IFN-γ and IL-4 secretion were analyzed by ELISA. E–J, Inhibition of glycolysis impairs allergic airway inflammation. WT mice were injected i.p with 2-DG (1.5 g/kg) or PBS (Mock), starting 1 day before OVA immunization until 1 day before sacrifice as indicated (E). Total BAL cells and differential cell counts (F), representative Kwik-Diff staining for BAL cytospins (G) and H&E staining of lung tissue sections (H), levels of cytokines and eotaxin in BAL fluids (I), and/or mRNA expression of cytokines and MUC-5AC in lung tissues (J) are shown. Results are representative of two (A–D) independent experiments. Error bars represent SD of 4–10 mice (A–D) or SE of 4 mice (F, I and J). *P < .05, **P < .01.