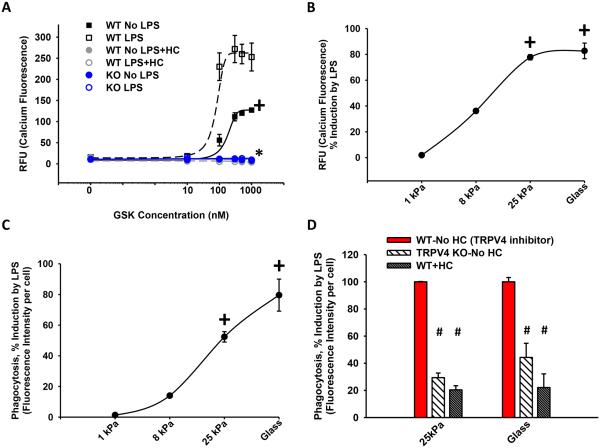
Figure 4. TRPV4 mediates the stiffness induction effect on LPS-stimulated calcium influx and macrophage phagocytosis.
BMDMs were treated ± LPS while attached to fibronectin-coated glass (50 × 106 GPa) (A) or polyacrylamide hydrogels of indicated stiffnesses (B-D). (A) Calcium influx was measured as in Figure 1B in WT BMDMs ± LPS ± HC versus KO BMDMs on glass substrate. LPS stimulated an increase in calcium influx that was abrogated with inhibition of TRPV4 (HC) or deletion of TRPV4 (KO) (*,+p < 0.05). (B) Calcium influx and (C) LPS-stimulated phagocytosis of E. coli particles, measured as % induction by LPS, were dependent on pathophysiologic range stiffness (>8-25 kPa) (+p < 0.05 ± LPS). (D) LPS-enhanced phagocytosis in WT BMDMs was decreased 4-fold upon deletion of TRPV4 (KO) or inhibition of TRPV4 (HC) on pathophysiologic range stiffness (25 kPa) (#p < 0.05). * denotes difference as compared to ± LPS, + denotes the increase in LPS vs UT (A) or difference consistent with 1 kPa (B-C), # denotes difference as compared to LPS treated WT ± HC (WT-No HC). n ≥ 3 times in quadruplicate.