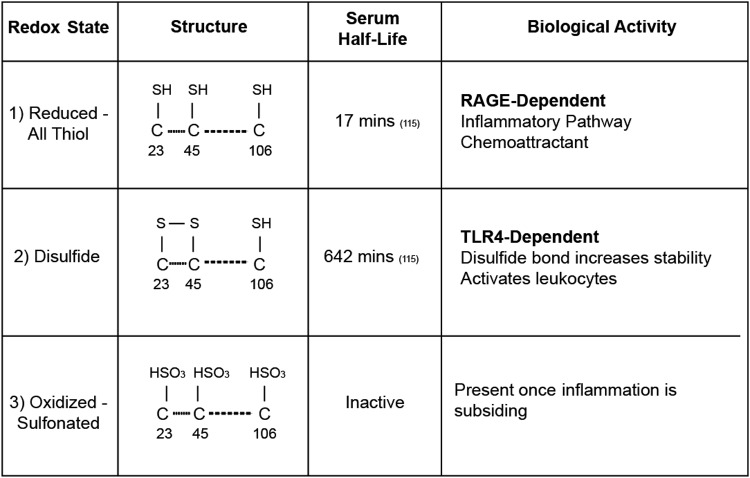
FIG. 3.
HMGB1 redox states and associated biological activity. The three redox states of HMGB1 differ by structure, half-life, and activity. The reduced form contains a thiol group at all three cysteine residues with a serum half-life of 17 min. It is this form that is able to bind to RAGE and activate inflammatory pathways. The disulfide form contains a disulfide bond between the cysteine residues at positions 23 and 45. This bond increases it stability and half-life to 642 min. Additionally, this is the form that is TLR4 dependent. The final inactivated form is known as the oxidized form and has sulfonated cysteines at all three positions. This is the predominant form once inflammation has begun to subside.