Abstract
Many comparative and developmental psychologists believe that we are Homo imitans; humans are more skilled and prolific imitators than other animals, because we have a special, inborn ‘intermodal matching’ mechanism that integrates representations of others with representations of the self. In contrast, the associative sequence learning (ASL) model suggests that human infants learn to imitate using mechanisms that they share with other animals, and the rich resources provided by their sociocultural environments. This article answers seven objections to the ASL model: (i) it presents evidence that newborns do not imitate; (ii) argues that infants receive a plentiful supply of the kind of experience necessary for learning to imitate; (iii) suggests that neither infants nor adults can imitate elementally novel actions; (iv) explains why non-human animals have a limited capacity for imitation; (v) discusses the goal-directedness of imitation; (vi) presents evidence that improvement in imitation depends on visual feedback; and (vii) reflects on the view that associative theories steal ‘the soul of imitation’. The empirical success of the ASL model indicates that the mechanisms which make imitation possible, by aligning representations of self with representations of others, have been tweaked by cultural evolution, not built from scratch by genetic evolution.
Keywords: active intermodal matching, associative learning, associative sequence learning, correspondence problem, imitation
1. Introduction
In everyday English, ‘imitation’ refers to all kinds of copying, from choosing a house in the same neighbourhood to mimicking a facial expression. But, in many areas of psychology and neuroscience, and in this article, ‘imitation’ is reserved for cases where there is a topographic resemblance between the behaviour of the copier (or ‘observer’ or ‘self’) and the agent who is copied (‘model’, ‘other’); where the parts of the observer's body move in the same way relative to one another as the parts of the model's body. For example, the eyebrows move upwards relative to the eyes, or the torso swivels relative to the hips. This kind of copying is of special interest because it is necessary for the development of culture-specific skills such as communicative gestures and ritualistic body movements [1], and promotes cooperative social interaction [1,2]. Topographic copying is also important because, in many cases, it has proved remarkably difficult to explain how representations of the self could be aligned with representations of the other in ways that make it possible to imitate. Imitation presents a self-other ‘correspondence problem’ [3]. Focusing on the imitation of facial gestures, Meltzoff & Moore [4, p. 179] elucidated this problem: ‘Infants can see the adult's face but cannot see their own faces. They can feel their own faces move, but have no access to the feelings of movement in the other. By what mechanism can they connect the felt but unseen movements of the self with the seen but unfelt movements of the other?’
Psychologists, who have been working on the correspondence problem for nearly a century, have come up with two kinds of solution: transformational and associative [5]. Transformational theories suggest that the problem is solved by mechanisms that convert a visual representation of the model's action, derived from observation, into a ‘symbolic’ or ‘intermodal’ representation, and that this intermediate (neither visual nor motor) representation enables observers both to produce the same actions as the model and, as a means to this end, to recognize the similarity between their own actions and those of the model. In contrast, associative theories suggest that the correspondence problem is solved by direct connections between visual and motor representations of action, and these connections enable observers to produce the same actions as the model ‘blindly’, without explicitly representing the relationship—of similarity or dissimilarity—between the model's and the observers' actions. In recent years, transformational and associative theories have been opposed, not only in the study of imitation, but also in research on the origin and function of mirror neurons. In this case, the ‘adaptation’ or ‘genetic’ account offers a transformational theory of mirror neurons [6–8].
In contemporary research on imitation, the dominant transformational theory is Meltzoff & Moore's [4] active intermodal matching (AIM) model, and the leading associative theory is the associative sequence learning (ASL) model [9,10]. AIM suggests that, when it comes to imitation, there is a fundamental discontinuity between humans and other animals. We are ‘Homo imitans’ [11]. Humans are more skilled and more prolific imitators than any other animals because only humans have an inborn, genetically inherited ‘module’ for imitation: an intermodal matching mechanism that can map representations of the self on to representations of others. In contrast, ASL suggests that there is continuity between imitation in human and non-human animals. Human infants learn to imitate using associative mechanisms that we share with other animals, and our prodigious imitative capacity is due primarily to the rich resources provided by our sociocultural environments (see reason IV below).
The ASL model differs from previous associative theories of imitation in several respects: (i) It is not ‘behaviourist’. The ASL model assumes that behaviour is caused by internal representations—percepts, memories, motor programmes—and learning constitutes changes in internal representation that can be inferred from, but are not always manifest in, behaviour. (ii) It distinguishes ‘vertical associations’, direct connections between visual and motor representations that solve the correspondence problem, from ‘horizontal associations’, connections among visual representations and among motor representations that, respectively, enable the observer to recognize and to perform new sequences of actions. (iii) It suggests that vertical associations are built by contingent visuomotor experience. For example, an infant's visual representation of mouth opening (MO) becomes connected with her motor representation of MO when, in a certain context, she is more likely to see MO than other events around the same time that she is performing MO. Reinforcement for MO is not necessary for the establishment of a vertical association. (iv) The ASL model identifies a number of sources in everyday life of the contingent visuomotor experience necessary for the formation of vertical associations: mirror self-observation, being imitated by others, synchronous action (when two or more agents respond at the same time, and in the same way, to an external stimulus) and acquired equivalence experience (e.g. hearing the same ‘popping’ sound when I open my mouth and, on other occasions, when I observe you opening your mouth). (v) The ASL model has been supported by a wide range of experiments with adult participants using behavioural and neurophysiological measures [10].
In spite of the ASL model's empirical support, the suspicion lingers in the minds of many that something as important and intriguing as imitation couldn't possibly be based on simple associative mechanisms. This article examines seven objections to the ASL model that have been raised in recent years. It argues in each case that, although the objection is initially appealing, it does not stand up against the evidence from studies of imitation in both human infants and non-human animals.
2. Reason I: Newborns can imitate
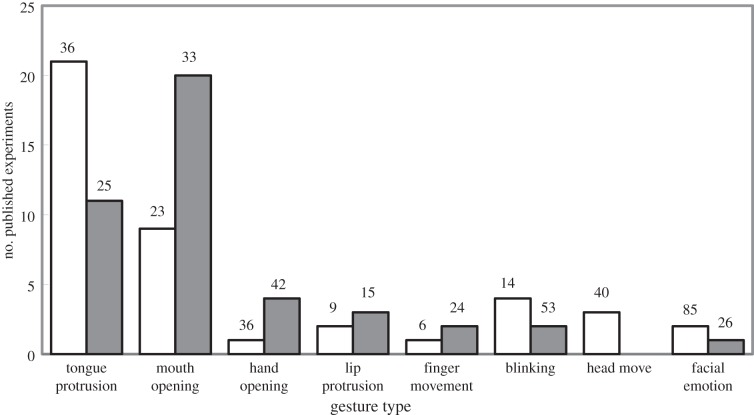
Newborns have had relatively little opportunity to learn. Therefore, if newborns could imitate a range of gestures, it would imply that imitation is mediated, not by learned vertical associations, but by some kind of inborn mechanism. However, in common with previous surveys [12], a recent, comprehensive review of research on imitation in newborns [13] found a reliable matching effect only for one gesture—tongue protrusion—and evidence that this effect does not have the specificity one would expect of imitation [14,15]. As figure 1 illustrates, for most of the other gestures that have been tested, the number of published experiments reporting failure to find imitation in newborns exceeds the number reporting positive evidence.
Figure 1.
Summary of experiments seeking evidence of gesture imitation in human newborns up to six-weeks old (adapted from Ray & Heyes [13]). ‘Gesture type’ refers to the target or modelled movement. Open bars indicate the number of published experiments reporting positive cross-target comparisons (i.e. infants performed the target action more often after observing the target action than after observing an alternative action). Filled bars indicate the number of published experiments reporting failure to find a significant difference in cross-target comparison. The number above each bar indicates average sample size.
Simpson et al. [16] suggested that this measure, the number of published experiments, gives a misleading impression because it does not take account of the sample size in each study. It is certainly not sufficient simply to count experimental outcomes, which is why Ray & Heyes [13] provided detailed methodological analysis of the whole corpus of imitation experiments involving newborns. However, the specific concerns expressed by Simpson et al. were unfounded. As the numbers above the bars in figure 1 indicate, aside from tongue protrusion, the average sample size in experiments reporting negative results was generally higher than the average sample size in experiments reporting positive results. Counting published studies may be misleading, but in the opposite direction to that anticipated by Simpson et al.: owing to publication bias, it is likely that this method overlooks a large number of unpublished failures to find evidence of imitation in newborns [17].
On close examination, studies of non-human primates also suggest that neonatal imitation is not a reliable phenomenon. For example, Ferrari et al. [18] reported imitation of tongue protrusion and lip-smacking in 3-day-old monkeys, but the effects were not present on days 1, 7 and 14 post-partum, and it is not clear whether they were replicated in a subsequent study using a similar procedure [6,19].
3. Reason II: Infants do not get the right kind of experience
The ASL model suggests that imitation is made possible by vertical associations—direct, bidirectional excitatory links between visual and motor representations of the same action—which are established when the observer experiences a contingent relationship between seeing and doing the same action [13]. ASL's emphasis on contingency follows the discovery, nearly 50 years ago, that associative learning does not depend solely on contiguity. For two event representations to become connected, the learner must experience the corresponding events, not only close together in time (contiguity), but in a predictive relationship (contingency; [20]): where the probability of the second event is higher when the first event has occurred than when the first event has not occurred. This overcomes Piaget's [21] worry that associations could not provide a basis for imitation (or any other behaviour), because there would be too many of them. For example, if contiguity was sufficient, a motor representation of MO might become associated, not just with a visual representation of MO, but also with visual representations of every stimulus that infants had happened to look at while opening their mouths.
However, it might be thought that, because contingency is required for association, ASL has a complementary ‘erosion problem’. For example, although there are a variety of contexts in which doing MO predicts seeing MO, and vice versa (e.g. when infants are being imitated by adults, or looking in a mirror, while repeatedly opening their mouths), these are not the only circumstances in which infants open their mouths. Infants also engage in MO while, for example, looking at their hands or at toys, and when their eyes are closed. Would not these episodes, when the infant is doing MO without seeing MO, erode the contingency between seeing and doing, and thereby prevent the formation of a vertical association?
This is a coherent and interesting objection but it overlooks a fundamental fact about associative learning: it occurs in a context, not in a vacuum. When a contingency between two events is experienced in one context but not in another—for example, when the lights are on, but not in the dark—the more enduring, background stimuli that constitute the context are drawn into the learning process; they become part of the association itself, or capable of activating and deactivating the association between event representations [22,23]. This kind of contextual control is likely to be strong in the case of facial expressions, because the relevant contingencies—e.g. between doing and seeing MO—are typically experienced only in the presence of a highly salient contextual stimulus, i.e. a face occupying a large proportion of the visual field. Thus, associative learning theory predicts that, rather than preventing the formation of a vertical association, doing MO without seeing MO, for example while looking at hands or toys, would firmly attach the vertical association with the face context. Consequently, the impulse to imitate MO should be stronger when MO is presented as part of a face than when it is presented against a different background.
4. Reason III: What about imitation of novel actions?
Actions vary on many dimensions and therefore they can be ‘novel’ in a variety of ways. For example, an action can be ‘new’ in the sense that it is performed faster than on previous occasions, with greater force, at a new location, or with novel topography, i.e. new spatial relations among different parts of the body. Two kinds of topographic novelty, sequential and elemental, are especially interesting in relation to imitation. In the former case, action units, characterized by their topography, are performed in a new combination or sequence. For example, if I had in the past touched the tip of my right thumb to the tip of each finger on my right hand, but never done so in the order ring–index–middle–little, then this sequence of thumb to finger contacts would be sequentially novel. In the case of elemental novelty, a new topographic relation is formed within an action unit. For example, I might touch the tip of my right index finger with the tip of my right little finger for the very first time.
ASL explains the imitation of sequentially novel actions with reference to horizontal associations, and this aspect of the model has not been challenged. However, some researchers have suggested that infants can imitate elementally novel actions, and that this shows that the ASL model must be wrong.
It is certainly true that it would be difficult for ASL to explain convincing evidence of the imitation of elementally novel actions. The model says that, in order to imitate an action unit, X, the observer must have a vertical association for X, and the formation of a vertical association for X requires the observer to perform X. Thus, in order to imitate X now, the observer must have done X at least once before. However, there is no compelling evidence that infants—or even adults—are able to imitate elementally novel actions.
One of the most widely cited potential examples of the imitation of an elementally novel action was presented by Meltzoff & Moore [24]. They reported that six-week-old infants imitated ‘TPside’, i.e. protrusion and retraction of the tongue between the lips at an angle to the body midline. Their data showed that, on the third of three test days, infants who had seen an adult performing TPside were more likely to make at least one TPside response than infants, combined for analysis, who had seen an adult performing TPmid (protrusion of the tongue along the body midline), performing MO or making no oral movements. This is an interesting result, but it needs to be interpreted with caution for several reasons: it comes from a single study; the effect was found only on 1 of 3 days of testing (see reason VI below); it was not reliable when the infants who had observed TPside were compared directly with those who had observed TPmid; it was not reflected in TPside frequency data; and the article did not report inter-rater reliability for TPside versus TPmid judgements or, crucially, any evidence that TPside was an elementally novel action for the infants who were supposed to have imitated TPside responses. Therefore, to provide sound evidence of the imitation of an elementally novel action, Meltzoff and Moore's result would need to be replicated with much more robust methods.
Another potential example comes from recent studies suggesting that infants can imitate bowing, i.e. bending from the waist, so that the head moves forward and down relative to the rest of the body [25–27]. It has been suggested that ASL cannot explain infant imitation of bowing, because the act of bowing typically makes the actor look down towards the floor. Therefore, even if infants bow while confronting a mirror, or in the presence of another agent who is also bowing, they are unlikely to see the bowing action—to receive the kind of contingent visual experience necessary for the formation of a vertical association relating to bowing.
This is an ingenious argument but, to be convincing, it needs to be supported by evidence. Specifically, we need to find out whether infants really do look down whenever they bow in everyday life, and whether they imitate bowing. At present, there is evidence that infants copy a ‘head-touch’ response: under certain conditions, observation of an adult bending from the waist to touch a light box with her forehead increases the probability that infants will also bend their heads [25]. However, this could be ‘effector matching’ rather than ‘movement matching’ (i.e. imitation), two phenomena that have been clearly dissociated in adult participants [28,29]. In other words, observation of bowing may not encourage infants specifically to produce bowing actions. Rather, observation of large head movements—any large head movements—may increase the probability that infants will move their own heads, and thereby the likelihood that, when they try to make contact with a desirable object—the light box—they will be scored as having done it with their heads [30].
In summary, the imitation of elementally novel actions, by infants or any other kind of agent, would present an interesting challenge for the ASL model, but there is currently no evidence that any agent is capable of this kind of imitation.
5. Reason IV: Other animals cannot imitate
ASL suggests not only that humans learn to imitate, but that we do not use any fancy, specialized cognitive machinery to do this learning. All that is needed ‘on the inside’ is a domain-general, taxon-general capacity for associative learning; the very same kind of learning that produces Pavlovian and instrumental conditioning in the laboratory. But if the development of imitation depends on associative learning, and the capacity for associative learning is taxon-general—present in a broad range of vertebrate and invertebrate species [31]—why are not all animals expert imitators?
There are two reasons: first, other animals lack resources ‘on the outside’. For example, they do not have optical mirrors; action words to provide acquired equivalence experience; extended periods of development in which, at least in some cultures, adults regularly imitate juveniles; or the kinds of rituals, drills and games—often involving music and dance—which provide humans with rich opportunities to see and do the same actions contingently. Some human rituals and games may even have the function of promoting the development of imitation; they may have culturally evolved for the ‘purpose’ of expanding the range of action units that children can imitate [1,32]. Second, other animals are much more limited in their capacity to encode novel sequences of stimuli, and therefore, in their ability to form horizontal associations among visual representations of action [33]. Consequently, even when they get the experience necessary to establish vertical associations, and thereby to solve the correspondence problem, animals are limited in their capacity to imitate new sequences of action.
However, there is now ample evidence that other animals have some capacity for imitation, and this evidence is consistent with ASL. It suggests that the development of imitation in non-human primates is highly experience-dependent [34,35] and, even in species that are distantly related to humans, such as birds, imitation is not merely a ‘trick’, but based on the same psychological mechanisms as human imitation. For example, Richards et al. [36] gave observer budgerigars access to a stopper immediately or 24 h after the observers had watched a video of a conspecific model either pecking or stepping on the stopper to obtain access to food. The observers performed the action they had observed, pecking or stepping, with higher frequency in both tests, suggesting that budgerigars are capable of ‘deferred imitation’ or ‘imitation from memory’, a capacity previously thought to be uniquely human [24].
6. Reason V: Imitation can be goal-directed
Sometimes, even adult humans imitate automatically; without intending to imitate, and in ways that interfere with ongoing tasks [37]. However, human imitation can also be highly selective and goal-directed. Actions that are observed to have happy results for the model—to be rewarded—are more likely to be imitated by both children and adults than actions that had unhappy results—the omission of reward, or the delivery of punishment—when performed by the model [38].
The duality of imitation, the fact that it is sometimes automatic and sometimes goal-directed, is entirely compatible with ASL because this model is built on contemporary associative learning theory, not on stimulus–response (S–R) behaviourism [39,40]. The latter suggested that all learning involves the formation of associations between stimuli (e.g. the sight of MO) and responses (e.g. the performance of MO), such that, whenever the stimulus is encountered, the response will be produced automatically. In contrast, contemporary associative learning theory (i) recognizes that S–R learning makes overt production of the response likely but not inevitable when the stimulus is encountered, and (ii) sees S–R learning as just one part of the associative learning story. When we make or observe an action (R) with an outcome (S*) in a context (S), we (adults, infants, non-human animals) learn, not only what to do in this context (S–R associations), but what outcome that action is likely to have (R–S*; [40]). Consequently, although ASL suggests that vertical associations are formed through S–R (and R–S) learning, it does not imply that observation of an action for which the observer has a vertical association will always and everywhere ignite an overt imitative response. Rather, once a vertical association is in place, observation of an action will activate a corresponding motor representation, producing a conscious or unconscious ‘urge’ to produce the action, but this urge can be inhibited or facilitated according to what the observer knows—via R–S* learning and by other means—about their situation and the likely consequences of performing the action.
More specifically, the covert activation of motor representations via vertical associations enables observational learning, i.e. learning about action outcomes by observation of their consequences when performed by a model. Consider, for example, an experiment by Akins & Zentall [41,42] in which Japanese quail observed conspecific models pecking or stepping on a pedal, when pedal depression resulted in the delivery of food to the model or had no programmed consequences. In a subsequent test, the observers imitated their models’ pecking or stepping action only if they had seen the behaviour rewarded. This result indicates that imitation in birds can be goal-directed, and thereby supports ASL by suggesting that complex psychological processes are not required for goal-directed imitation. ASL proposes that the quails' imitative behaviour was sensitive to model reward, because, during observation, the sight of the model's response activated a motor representation of the same response in the observer (by virtue of a vertical association), and this motor activation predicted an event that was rewarding for the observer, i.e. the sight of food and/or feeding. Consequently, the observers in the model rewarded group learned an R–S* association between the modelled action and its consequences, which promoted performance of imitative responses on test.
Thus, the fact that imitation can be goal-directed, in human and non-human animals, does not present a challenge to the ASL model.
7. Reason VI: Imitation improves without visual feedback
Associative theories of imitation suggest that the correspondence problem is solved by a process that does not calculate or explicitly represent the topographic similarity between the model's and the observer's actions; the similarity between the other and the self. When the visual and motor representations connected by a vertical association are representations of the same action, activation of the visual representation by observation of a model's action is apt to produce a topographically similar response by the observer; to solve the correspondence problem. But, the process—the transfer of excitation from a visual to a motor representation via a learned association—does not ‘know’ that the visual and motor representations are of topographically equivalent actions. It would proceed in exactly the same way if, as a result of prior learning, the association was between a visual representation of one action, say MO, and a motor representation of a different action, for example lip protrusion. In contrast, transformational theories of imitation suggest that the correspondence problem is solved by a process that somehow computes the degree of topographic similarity between the model's and the observer's actions. For example, Meltzoff and Moore's transformational theory suggests that the AIM mechanism represents the ‘the equivalence between the acts of self and other’ [43]; it compares the topography of the model's action with the topography of the infant's early attempts to copy the model's action, and guides the infant's further attempts by progressively reducing the disparity between the two topographies.
In support of the AIM proposal, Meltzoff & Moore [24] reported that, with successive opportunities to observe an adult performing TPside, and to make responses, infants tended to produce actions that were increasingly similar to TPside. The infants in this experiment were not selectively rewarded for responses approximating to TPside, and they did not receive visual feedback; they could not see their own responses. Therefore, if the infants' imitation of TPside really did improve over trials, it would suggest that AIM is right: infant imitation is guided by a mechanism that can compute, and progressively maximize, the topographic similarity between the model's and observer's action. But, it is not clear whether the infants' imitation really did improve over trials. Meltzoff and Moore analysed their data in a way that left open the possibility that their infants' simply made larger, more vigorous, responses as they became more familiar with the testing situation, or more aroused by repeated presentation of TP [12,13].
The results of a recent experiment with adult participants [44] suggest that the infants tested by Meltzoff and Moore were responding more vigorously rather than improving their imitation. In the adult study, participants were shown a target facial expression, and allowed a series of attempts to imitate the target, with explicit instructions to improve their imitative performance over trials. This experiment used for the first time precise, fully automated procedures to measure imitation. These procedures revealed that adults who were given the task with visual feedback—allowed to see each of their attempts to imitate—showed steady improvement over trials. However, adults who did not receive visual feedback—who were tested under the same conditions as Meltzoff and Moore's infants—showed no improvement at all. In accord with ASL, this outcome suggests that the mechanism that solves the correspondence problem does not compute the topographic similarity between the model's and the observer's actions. Adults may have other, language-based mechanisms that allow them to make explicit judgements about the topographic relations between different actions, but the results reported by Cook et al. suggest that these are not the mechanisms that solve the correspondence problem for imitation.
8. Reason VII: Associative explanations steal ‘the soul of imitation’
The final objection to be considered in this article may be the most important, the greatest obstacle faced by associative accounts of imitation, but it is also the hardest to pin down: many researchers feel that associative explanations are boring; they take the magic out of that which they explain. For example, Meltzoff [43, p. 55] has suggested that, when a solution to the correspondence problem does not appeal to mechanisms computing the similarity between observed and executed actions, ‘the soul of imitation has been snatched away’.
I suspect that no amount of argument or evidence could counteract this feeling that ASL, or any associative account, is a bad fairy threatening to steal the soul of imitation. It is a matter of intellectual taste. The suggestion that simple mechanisms can produce subtle and complex psychological phenomena is, for some researchers, brash and disenchanting. For others, like me, it is exhilarating; it suggests that Mother Nature can build grand edifices out of simple, uniform blocks, and that—if we are willing to tackle deep conceptual and methodological problems—fascinating, scientific mysteries can be penetrated. Rather than argue logically for the latter view, all I can do is describe what the explanatory landscape looks like from this side, where associative explanations are appealing. From here, the dominant transformational theory of imitation, the AIM model, looks like an unsatisfactory ‘Chinese box explanation’ [45]. In Moliere's play, The imaginary invalid, physicians ‘explain’ the power of opium to induce sleep by saying that it has ‘dormitive virtue’; it contains something, the nature of which is unspecified, that causes sleep. Similarly, AIM ‘explains’ the power of observers to solve the correspondence problem by saying that they have ‘AIM’: something inside, the nature of which is unspecified, that solves the correspondence problem. The AIM model tells us that this ‘something inside’ computes topographic similarity, but it does not tell us how it performs these computations. It tells us where (inside) but not how the correspondence problem is solved. This explanatory strategy may ‘save the soul of imitation’, but isn't science about solving, rather than preserving, mysteries?
9. Conclusion
Humans are gifted imitators, and imitation plays an important part in making human minds and human lives very different from those of other animals. Imitation enables us to acquire gestures and skills that mark us out as members of particular cultural groups, gives us a sense of belonging, promotes cooperation and contributes to cultural evolution, the accumulation of knowledge and improvement of skills over generations [46]. When humans are especially good at something, compared with other animals, and when our skill has important consequences, there is a strong temptation to assume that it must be underwritten by very special psychological processes; that genetic evolution has given us a way of thinking that is completely absent in other animals. The ASL model, defended here, suggests that this is not true of imitation. The mechanisms that make imitation possible, by aligning representations of self with representations of others, are associative mechanisms that we share with a great many other animals. Our prodigious capacity for imitation is due to tweaking rather than wholesale reconstruction [30]. Genetic and cultural evolution have added some domain-general capacity for sequence processing, and cultural evolution has enriched the environment in which humans learn to imitate, but the basic mechanisms that solve the correspondence problem—that link self with other for imitation—are the same in us as they are in ‘dumb’ animals. So, are we Homo imitans? Yes, in that we are great imitators. No, because we are made that way, not by Mother Nature, but by learning and culture.
Acknowledgements
I am grateful to the editors of this theme issue, two anonymous referees for their constructive comments, and to all those who participated in the debate on ‘Imitation and the mirror neuron system’ at the International Conference on Infant Studies, Berlin, 2014, especially Harold Bekkering, Gyorgy Gergely, Mark Johnson, Carina de Klerk, Denis Mareschal and Andrew Meltzoff. Thanks also to Richard Cook for his comments on an earlier draft, and for relinquishing his earlier, cherished conception of ‘meaning’.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Heyes C. 2013. What can imitation do for cooperation? In Cooperation and its evolution (eds Sterelny K, Joyce R, Calcott B, Fraser B), pp. 313–332. Cambridge, MA: MIT Press. [Google Scholar]
- 2.Chartrand TL, Bargh JA. 1999. The chameleon effect: the perception–behavior link and social interaction. J. Pers. Soc. Psychol. 76, 893 ( 10.1037/0022-3514.76.6.893) [DOI] [PubMed] [Google Scholar]
- 3.Brass M, Heyes C. 2005. Imitation: is cognitive neuroscience solving the correspondence problem? Trends Cogn. Sci. 9, 489–495. ( 10.1016/j.tics.2005.08.007) [DOI] [PubMed] [Google Scholar]
- 4.Meltzoff AN, Moore MK. 1997. Explaining facial imitation: a theoretical model. Early Dev. Parent. 6, 179–192. () [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyes C, Bird G. 2002. Transformational and associative theories of imitation. In Imitation in animals and artifacts (eds Dautenhahn K, Nehaniv C), pp. 501–523. Cambridge, MA: MIT Press. [Google Scholar]
- 6.Cook R, Bird G, Catmur C, Press C, Heyes C. 2014. Mirror neurons: from origin to function. Behav. Brain Sci. 37, 177–192. ( 10.1017/S0140525X13000903) [DOI] [PubMed] [Google Scholar]
- 7.Decety J, Meltzoff AN. 2011. Empathy, imitation, and the social brain. Empathy: Philos. Psychol. Perspect. 2011, 58–81. ( 10.1093/acprof:oso/9780199539956.003.0006) [DOI] [Google Scholar]
- 8.Heyes C. 2010. Where do mirror neurons come from? Neurosci. Biobehav. Rev. 34, 575–583. ( 10.1016/j.neubiorev.2009.11.007) [DOI] [PubMed] [Google Scholar]
- 9.Heyes C, Ray ED. 2000. What is the significance of imitation in animals? Adv. Stud. Behav. 29, 215–245. ( 10.1016/S0065-3454(08)60106-0) [DOI] [Google Scholar]
- 10.Catmur C, Walsh V, Heyes C. 2009. Associative sequence learning: the role of experience in the development of imitation and the mirror system. Phil. Trans. R. Soc. B 364, 2369 ( 10.1098/rstb.2009.0048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meltzoff AN. 1988. The human infant as Homo imitans. In Social learning: psychological and biological perspectives (eds TR Zentall, BG Galef), pp. 319–341. Hillsdale, NJ: Erlbaum. [Google Scholar]
- 12.Anisfeld M. 2005. No compelling evidence to dispute Piaget's timetable of the development of representational imitation in infancy. Perspect. Imit. 2, 107–131. [Google Scholar]
- 13.Ray E, Heyes C. 2011. Imitation in infancy: the wealth of the stimulus. Dev. Sci. 14, 92–105. ( 10.1111/j.1467-7687.2010.00961.x) [DOI] [PubMed] [Google Scholar]
- 14.Jones SS. 2006. Exploration or imitation? The effect of music on 4-week-old infants’ tongue protrusions. Infant Behav. Dev. 29, 126–130. ( 10.1016/j.infbeh.2005.08.004) [DOI] [PubMed] [Google Scholar]
- 15.Johnson MH, Senju A, Tomalski P. 2014. The two-process theory of face processing: modifications based on two decades of data from infants and adults. Neurosci. Biobehav. Rev. 50, 169–179. ( 10.1016/j.neubiorev.2014.10.009) [DOI] [PubMed] [Google Scholar]
- 16.Simpson EA, Murray L, Paukner A, Ferrari PF. 2014. The mirror neuron system as revealed through neonatal imitation: presence from birth, predictive power and evidence of plasticity. Phil. Trans. R. Soc. B 369, 20130289 ( 10.1098/rstb.2013.0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson CJ, Heene M. 2012. A vast graveyard of undead theories: publication bias and psychological science's aversion to the null. Perspect. Psychol. Sci. 7, 555–561. ( 10.1177/1745691612459059) [DOI] [PubMed] [Google Scholar]
- 18.Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. 2006. Neonatal imitation in rhesus macaques. PLoS Biol. 4, e302 ( 10.1371/journal.pbio.0040302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paukner A, Ferrari PF, Suomi SJ. 2011. Delayed imitation of lipsmacking gestures by infant rhesus macaques (Macaca mulatta). PLoS ONE 6, e28848 ( 10.1371/journal.pone.0028848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rescorla RA. 1968. Probability of shock in the presence and absence of CS in fear conditioning. J. Comp. Physiol. Psychol. 66, 1 ( 10.1037/h0025984) [DOI] [PubMed] [Google Scholar]
- 21.Piaget J. 1952. Play, dreams and imitation in childhood. New York, NY: Norton. [Google Scholar]
- 22.Wilson PN, Pearce JM. 1989. A role for stimulus generalization in conditional discrimination learning. Q. J. Exp. Psychol. 41, 243–273. [PubMed] [Google Scholar]
- 23.Cook R, Dickinson A, Heyes C. 2012. Contextual modulation of mirror and countermirror sensorimotor associations. J. Exp. Psychol. Gen. 141, 774–787. ( 10.1037/a0027561) [DOI] [PubMed] [Google Scholar]
- 24.Meltzoff AN, Moore MK. 1994. Imitation, memory, and the representation of persons. Infant Behav. Dev. 17, 83–99. ( 10.1016/0163-6383(94)90024-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gergely G, Bekkering H, Király I. 2002. Rational imitation in preverbal infants. Nature 415, 755 ( 10.1038/415755a) [DOI] [PubMed] [Google Scholar]
- 26.Paulus M, Hunnius S, Vissers M, Bekkering H. 2011. Imitation in infancy: rational or motor resonance? Child Dev. 82, 1047–1057. ( 10.1111/j.1467-8624.2011.01610.x) [DOI] [PubMed] [Google Scholar]
- 27.Paulus M, Hunnius S, Vissers M, Bekkering H. 2011. Bridging the gap between the other and me: the functional role of motor resonance and action effects in infants’ imitation. Dev. Sci. 14, 901–910. ( 10.1111/j.1467-7687.2011.01040.x) [DOI] [PubMed] [Google Scholar]
- 28.Leighton J, Heyes C. 2010. Hand to mouth: automatic imitation across effector systems. J. Exp. Psychol. Hum. Percept. Perform. 36, 1174–1183. ( 10.1037/a0019953) [DOI] [PubMed] [Google Scholar]
- 29.Gillmeister H, Catmur C, Liepelt R, Brass M, Heyes C. 2008. Experience-based priming of body parts: a study of action imitation. Brain Res. 1217, 157–170. ( 10.1016/j.brainres.2007.12.076) [DOI] [PubMed] [Google Scholar]
- 30.Heyes C. In press Born pupils? Natural pedagogy and cultural pedagogy. Perspect. Psychol. Sci. [DOI] [PubMed] [Google Scholar]
- 31.Heyes C. 2012. Simple minds: a qualified defence of associative learning. Phil. Trans. R. Soc. B 367, 2695–2703. ( 10.1098/rstb.2012.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomasello M. 2014. A natural history of human thinking. Cambridge, MA: Harvard University Press. [Google Scholar]
- 33.Conway CM, Christiansen MH. 2001. Sequential learning in non-human primates. Trends Cogn. Sci. 5, 539–546. ( 10.1016/S1364-6613(00)01800-3) [DOI] [PubMed] [Google Scholar]
- 34.Custance DM, Whiten A, Bard KA. 1995. Can young chimpanzees (Pan troglodytes) imitate arbitrary actions? Hayes & Hayes (1952) revisited. Behaviour 132, 837–859. ( 10.1163/156853995X00036) [DOI] [Google Scholar]
- 35.Tomasello M, Savage-Rumbaugh S, Kruger AC. 1993. Imitative learning of actions on objects by children, chimpanzees, and enculturated chimpanzees. Child Dev. 64, 1688–1705. ( 10.2307/1131463) [DOI] [PubMed] [Google Scholar]
- 36.Richards C, Mottley K, Pearce J, Heyes C. 2009. Imitative pecking by budgerigars, Melopsittacus undulatus, over a 24 h delay. Anim. Behav. 77, 1111–1118. ( 10.1016/j.anbehav.2009.01.019) [DOI] [Google Scholar]
- 37.Heyes C. 2011. Automatic imitation. Psychol. Bull. 137, 463–483. ( 10.1037/a0022288) [DOI] [PubMed] [Google Scholar]
- 38.Bandura A, Ross D, Ross SA. 1963. Vicarious reinforcement and imitative learning. J. Abnorm. Soc. Psychol. 67, 601–607. ( 10.1037/h0045550) [DOI] [PubMed] [Google Scholar]
- 39.Klossek UMH, Dickinson A. 2012. Rational action selection in 1½-to 3-year-olds following an extended training experience. J. Exp. Child Psychol. 111, 197–211. ( 10.1016/j.jecp.2011.08.008) [DOI] [PubMed] [Google Scholar]
- 40.Dickinson A. 1985. Actions and habits: the development of behavioural autonomy. Phil. Trans. R. Soc. Lond. B 308, 67–78. ( 10.1098/rstb.1985.0010) [DOI] [Google Scholar]
- 41.Akins CK, Zentall TR. 1998. Imitation in Japanese quail: the role of reinforcement of demonstrator responding. Psychon. Bull. Rev. 5, 694–697. ( 10.3758/BF03208847) [DOI] [Google Scholar]
- 42.Saggerson AL, George DN, Honey RC. 2005. Imitative learning of stimulus-response and response-outcome associations in pigeons. J. Exp. Psychol. Anim. Behav. Process. 31, 289–300. ( 10.1037/0097-7403.31.3.289) [DOI] [PubMed] [Google Scholar]
- 43.Meltzoff AN. 2005. Imitation and other minds: the ‘like me’ hypothesis. Perspect. Imit. 2, 55–77. [Google Scholar]
- 44.Cook R, Johnston A, Heyes C. 2013. Facial self-imitation objective measurement reveals no improvement without visual feedback. Psychol. Sci. 24, 93–98. ( 10.1177/0956797612452568) [DOI] [PubMed] [Google Scholar]
- 45.Sober E. 1982. Why must homunculi be so stupid? Mind 91, 420–422. ( 10.1093/mind/XCI.363.420) [DOI] [Google Scholar]
- 46.Over H. 2016. The origins of belonging: social motivation in infants and young children. Phil. Trans. R. Soc. B 371, 20150072 ( 10.1098/rstb.2015.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]