Abstract
We review the evidence that an ability to achieve a precise balance between representing the self and representing other people is crucial in social interaction. This ability is required for imitation, perspective-taking, theory of mind and empathy; and disruption to this ability may contribute to the symptoms of clinical and sub-clinical conditions, including autism spectrum disorder and mirror-touch synaesthesia. Moving beyond correlational approaches, a recent intervention study demonstrated that training participants to control representations of the self and others improves their ability to control imitative behaviour, and to take another's visual perspective. However, it is unclear whether these effects apply to other areas of social interaction, such as the ability to empathize with others. We report original data showing that participants trained to increase self–other control in the motor domain demonstrated increased empathic corticospinal responses (Experiment 1) and self-reported empathy (Experiment 2), as well as an increased ability to control imitation. These results suggest that the ability to control self and other representations contributes to empathy as well as to other types of social interaction.
Keywords: empathy, self–other control, transcranial magnetic stimulation, imitation–inhibition, motor-evoked potentials, social interaction
1. Introduction
Successful social interaction involves manipulating neural representations of other people across diverse domains including imitation, perspective-taking, theory of mind (ToM) and empathy [1]. In this article, we first review the evidence that success within each of these areas requires the ability to control the extent to which representations of the self or of the other are activated (‘self–other control’). We then discuss how a breakdown in this ability contributes to the symptoms of both clinical and sub-clinical conditions. In the third section, we present original data demonstrating that training self–other control in one domain of social cognition, imitation, has effects on performance in another domain, empathy, supporting the claim that self–other control contributes to social interaction across social domains.
2. Self–other control within social cognitive domains
When interacting with another person, we must process constantly changing social information including the actions, perspectives, beliefs and emotions of others. There is now compelling evidence that processing these attributes in another activates the same neural representations as when the self experiences these events (‘mirroring’ [2–4]), because of associations between other- and self-relevant representations [5,6]. Such ‘mirror’ processes result in potential conflict between self- and other-relevant representations, and thus a requirement for ‘self–other control’: the ability to manipulate the extent to which self- or other-relevant representations are activated. For example, the control of imitation requires the ability to distinguish between one's own motor plan and that of the other, instantiated in the self through psychological and neurological processes supporting imitation, before the other-related motor programme is inhibited and that relating to the self enhanced [7]. When taking another person's perspective, representation of one's own perspective must be inhibited in favour of representation of the other's perspective [8]. Similarly, in ToM tasks, one needs to represent the other's beliefs, rather than one's own [9]. Finally, when empathizing with another person, differential activation of self- or other-related representations may lead to different outcomes: in order to feel ‘with’ another, the affective state resulting from representation of the other's emotions must be enhanced and the representation of one's own affective state inhibited; however, in order to prevent excessive personal distress (PD) as a result of another's negative affective state, it may be more adaptive to inhibit the representation of the other's affective state [10]. Overall, it appears that a similar mechanism of self–other control contributes to successful performance within each of these social cognitive domains.
The suggestion that self–other control involves a common neural mechanism is supported by both neuroimaging and neurostimulation data indicating that the right temporoparietal junction (TPJ) supports a process which contributes to imitation control, perspective-taking and ToM [11–15]. However, the control of representations of the self and others in the domain of empathy depends on a different area of parietal cortex (supramarginal gyrus [16]). The finding that different areas of parietal cortex may support self–other control for different domains of social interaction (broadly, cognitive versus affective domains) suggests two contrasting scenarios. The first is that empathy may not involve the same self–other control process as imitation control, perspective-taking and ToM. Alternatively, these anatomically distinct areas of parietal cortex may implement the same cognitive process on different inputs. Under the first scenario, the ability to implement self–other control in one socio-cognitive domain should not be related to that ability in other domains; whereas under the second scenario, impairments in one domain might be expected to correlate with impairments in others. Therefore, the next section considers clinical and sub-clinical conditions in which self–other control may be impaired, and investigates whether impairments in one social domain correlate with those in others.
3. Impairments in self–other control
(a). Autism spectrum disorder
Primary evidence for impaired self–other control in autism spectrum disorder (ASD) is derived from the frequent difficulties experienced by autistic individuals when attempting to inhibit the tendency to imitate others. As discussed above, the ability to inhibit imitation and instead execute a task-appropriate action requires the control of other-related motor programs. It has been long been recognized clinically that individuals with ASD are less able to inhibit imitation of the speech (echolalia) [17] and actions (echopraxia) [18] of others, and recent experimental data confirm the reduced ability to inhibit imitation in individuals with ASD [1,19]. Reduced ability to control representations of the self and others may also contribute to the well-established impairments in ToM exhibited by individuals with ASD [20], such that representation of one's own mental state interferes with accurate representation of that of another, particularly during situations exemplified by False Belief tasks. Indeed, the degree to which individuals with ASD were impaired at imitation–inhibition has been shown to predict both performance on a behavioural (verbal) test of ToM and neurological activation within a previously identified ToM network when participants completed a non-verbal ToM task [1]. Direct evidence of atypical neurological activation during a self- and other-processing task was provided by Lombardo et al. [21,22], who demonstrated atypical response in TPJ, the area of the brain thought to be responsible for self–other control, when participants with ASD were asked to selectively represent the self or another.
More speculative is the suggestion that the increased PD in response to another's pain in ASD ([23]; see [24] for an overview) is a direct consequence of impaired self–other control. Several authors [25–27] have argued that responses to the pain of another can be considered on a maturational gradient determined by self–other control. Under these models, PD is considered to be an immature response to another's pain in which representation of the other's pain is unable to be inhibited, to the extent that the observer feels a significant degree of negative arousal. It is only later, when a sufficient degree of self–other control has been achieved, that PD reduces and empathic concern increases. This overview suggests that difficulties in self–other control may contribute to difficulties in the domains of imitation, ToM and empathic responses to another's pain in ASD.
(b). Mirror-touch synaesthesia
A further condition associating atypical socio-cognitive development with impaired self–other control is mirror-touch synaesthesia. Individuals with mirror-touch synaesthesia experience tactile sensations on their own body when observing touch to other individuals [28–30], and (less frequently) when observing touch to objects [29–31]. The experience has also been linked with broader differences in social perception and cognition, including heightened empathy [32,33] and emotion perception [34].
A recent explanation of mirror-touch synaesthesia suggests it results from difficulties in the ability to distinguish and control representations of the self from others [35]. This leads to the amplified vicarious tactile experiences symptomatic of the condition as a result of failure to inhibit other-relevant somatosensory representations. This explanation is supported by evidence that mirror-touch synaesthetes show structural brain differences which extend beyond brain regions involved in somatosensory mirroring to those involved in self–other control, including the right TPJ [36]. This account further predicts that mirror-touch synaesthetes should show impairments in other social cognitive domains where the control of other-relevant representations is required. Thus, it is noteworthy that mirror-touch synaesthetes show impairments in the ability to control imitation (requiring inhibition of other-relevant representations), but are not impaired at visual perspective-taking or ToM (requiring inhibition of self-relevant representations) [37], suggesting a specific impairment in self–other control processes in mirror-touch synaesthesia that may contribute to the documented atypical interpersonal representations of touch and emotion processing in this condition. This provides further evidence that self–other control may contribute to performance in multiple social cognitive domains.
4. Training self–other control across social cognitive domains
Sections 2 and 3 used task analysis along with patterns of anatomical and clinical correlation to argue that the same self–other control processes are involved across a variety of social domains. However, although these approaches are supportive of this conclusion, they do not demonstrate a causal link between self–other control ability and performance across social cognitive domains. An alternative approach to establish the contribution of self–other control to social interaction is to train this ability in one domain and assess the effect of training on other areas of social interaction. Santiesteban et al. [38] trained participants to increase self–other control in the motor domain, by means of a task based on the work of Brass et al. [39] requiring them to inhibit the tendency to imitate another person (increasing self–other control) or to enforce this tendency (decreasing self–other control). Training to increase self–other control improved both the control of imitation and visual perspective-taking. As noted above, however, both processes are known to rely on right TPJ [13,14]. A more stringent test of the involvement of self–other control across social cognitive domains is to measure whether training to increase self–other control in the motor domain affects a process that does not depend on the same neuroanatomical location, namely empathy. This study, therefore, tested whether training to increase self–other control influences empathy, using both an implicit corticospinal empathy measure [40] (Experiment 1) and an explicit self-report measure (Experiment 2).
A single transcranial magnetic stimulation (TMS) pulse applied to the primary motor cortex representation of a particular muscle produces a motor-evoked potential (MEP) in that muscle, the amplitude of which reflects corticospinal excitability. Observation of another person receiving a painful stimulus results in reduced MEP amplitude compared with observation of touch [40–43]. This corticospinal empathy measure has been interpreted as automatic simulation of another's pain because the same inhibition occurs during receipt of pain to the self [43], possibly reflecting a withdrawal reflex [44].
If self–other control contributes to empathy in the same way that it does to other areas of social interaction, then training should impact upon empathy, because participants trained to increase self–other control should be better able to separate their own, non-pain, state from the pain state of the other. This improved separation should mean that participants exhibit less of an egocentric bias when representing the other [16], and thus simulate their pain to a greater extent, increasing corticospinal inhibition during observation of pain in another person, compared with during observation of touch.
Accordingly, participants were randomly allocated to one of two training groups (increased and decreased self–other control). Twenty-four hours after training, corticospinal empathy and the ability to control imitation were measured and compared between groups (Experiment 1). In Experiment 2, training-related changes in self-reported empathy were measured.
(a). Experiment 1
(i). Methods
Participants
Twenty-six right-handed participants aged 18–42 years (mean 22.8) were recruited from the University of Surrey and screened for TMS contra-indications. To control for any effect of PD on corticospinal empathy (corticospinal empathic responses reverse in individuals with high levels of PD [41,45]) individuals were pre-screened using the Davis Interpersonal Reactivity Index (IRI) PD subscale [46]. Those with a PD score of 13 or lower (within 1 s.d. of the population mean [47]) were randomly assigned to either the decreased self–other control or increased self–other control training group. Participants attended on two consecutive days. One participant in the decreased self–other control group did not complete Day 2, resulting in 13 participants (five male) in the decreased self–other control group and 12 participants (five male) in the increased self–other control group. Groups did not differ in terms of age or IRI subscale scores (all ps > 0.3).
Procedure
On Day 1, participants received either imitation training or imitation–inhibition training to decrease or increase self–other control, respectively [38]. On Day 2, participants: (i) underwent single pulse TMS to assess corticospinal empathic responses to pain observation; (ii) completed an imitation control task, and (iii) completed a control simple response time task. Following testing, participants were debriefed. No participants reported awareness of the link between the Day 1 training and the Day 2 empathy test.
Day 1: training to decrease or increase self–other control
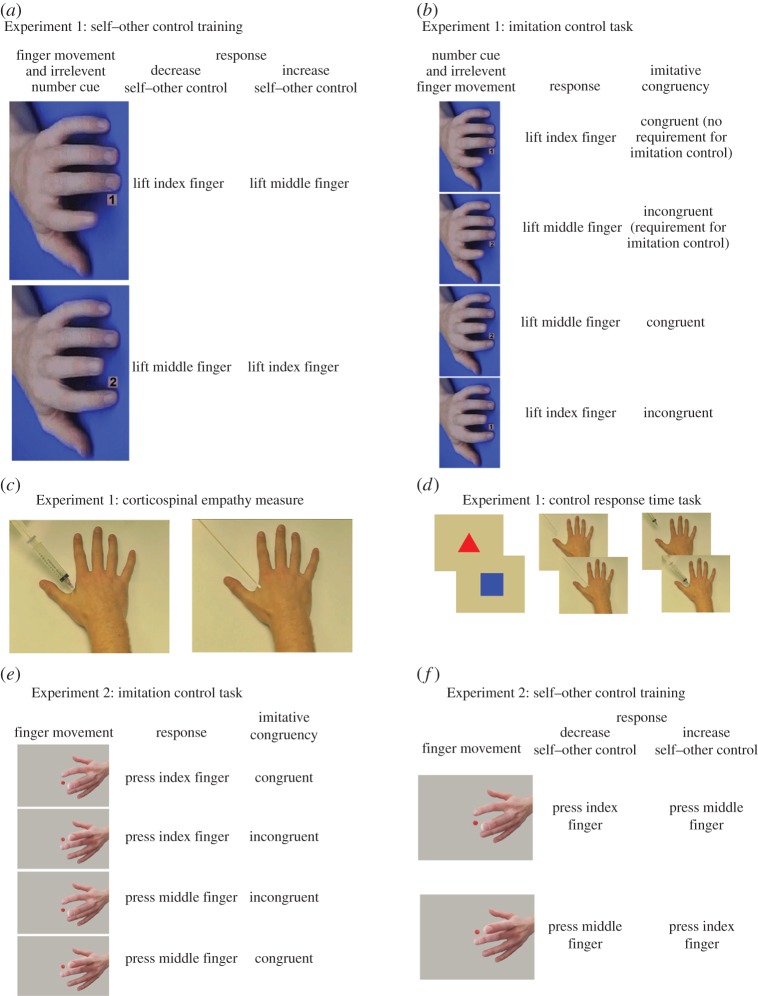
Participants in both groups performed a task based on that developed by Brass et al. [39]. Short videos were presented to participants with either an index or middle finger performing a lifting movement (figure 1a). A resting left hand was presented for a variable duration (800–2400 ms) before the onset of an irrelevant number (1 or 2) and a finger lifting movement, which lasted 68 ms with a final frame of 500 ms in which the finger remained in the lifted position. The stimulus hand was rotated around the sagittal and transverse planes with respect to the participant's right hand, which rested on the keyboard. This was to prevent any possible confounds because of spatial compatibility [48]. Participants were instructed to press their index finger down on the ‘V’ key and the middle finger down on the ‘B’ key. Participants in the decreased self–other control group were asked to lift their index finger when the index finger of the stimulus hand lifted, and to lift their middle finger when the middle finger of the stimulus hand lifted. Participants in the increased self–other control group were asked to lift their middle finger when the index finger of the stimulus hand lifted, and to lift their index finger when the middle finger of the stimulus hand lifted. Participants were instructed to press back down on the appropriate starting key before the following trial and to ignore the numbers, 1 or 2, that appeared on the screen. A total of 432 trials were presented randomly across six blocks, with a fully factorial combination of stimulus movement (index or middle finger lift) and irrelevant number (1 or 2) repeated 18 times per block for a total of 72 trials per block. Response times were measured from the onset of the finger movement and irrelevant number.
Figure 1.
Stimuli for Experiment 1 (a) self–other control training; (b) imitation control task; (c) measurement of corticospinal empathy; (d) response time task, and Experiment 2 (e) imitation control task; (f) self–other control training. (a) During self–other control training, participants either performed the same, or a different, movement to that observed according to their assigned training group, and ignored the number cues. Two of four possible trial types are shown here; two further trial types were also presented (index finger video with irrelevant number 2; middle finger video with irrelevant number 1). (b) During the imitation control task, all participants responded to the number cues while ignoring the finger movements. They were instructed to lift their index finger on presentation of a 1, and their middle finger on presentation of a 2. Trials on which the irrelevant finger movement is congruent with the cued response produce no requirement for imitation control; trials on which the irrelevant movement is incongruent with the cued response require the participant to control the tendency to imitate the irrelevant finger movement. (c) Final frames of the pain and touch videos. (d) Image pairs used in control response time task. (e) Stimuli used in imitation control task in Experiment 2. (f) Stimuli used in self–other control training in Experiment 2. (Online version in colour.)
Day 2: (i) Corticospinal empathy
TMS and MEP recordings: single-pulse TMS was administered using a Magstim Rapid2 stimulator with a 70 mm figure-8 coil positioned over left primary motor cortex, at the position from which MEPs with maximal amplitude could be recorded from both the first dorsal interosseus (FDI) target muscle and abductor digiti minimi (ADM) control muscle. Resting motor threshold (rMT) was determined as the lowest stimulus intensity that induced at least five MEPs (of at least 50 µV peak-to-peak amplitude) out of 10 consecutive TMS pulses in both muscles [49]. Mean rMT was 56.9% (range 44–74%) of maximum stimulator intensity. During the recording session, stimulation intensity was set to 120% of rMT. MEPs were recorded simultaneously from FDI and ADM muscles of the participant's right hand. Electromyographic recording was performed through pairs of Ag–AgCl surface electrodes placed over the muscle belly (active electrode) and the associated joint or tendon (reference electrode). The ground was placed over the participant's right wrist. The signal was sampled (5000 Hz), amplified, band-pass filtered (3 Hz–1000 Hz) with a 50 Hz notch filter and stored for off-line analysis. Data were collected from 100 ms before to 300 ms after the TMS pulse.
MEP analysis: trials with muscle activity greater than 50 µV in the 100 ms before the TMS pulse were discarded (mean ± s.e.m. percentage of trials in FDI: 1.3 ± 0.6%; ADM: 0.6 ± 0.3%). For each muscle, peak-to-peak MEP amplitudes for each trial were normalized to the block median MEP amplitude for that muscle. Extreme outlier trials (MEP amplitude greater than 3 s.d. from the mean for each muscle) were subsequently removed (FDI: 3.4 ± 0.6%; ADM: 3.6 ± 0.6%) and the mean normalized MEP amplitude was calculated for each condition in each muscle. Corticospinal empathy was calculated by subtracting mean normalized MEP size during the touch control condition from that during pain observation and dividing this value by that obtained during the baseline static hand condition [42].
Stimuli and procedure: stimulus videos were modelled on those used by Avenanti et al. [40]. The videos were 1800 ms in length and were presented from a first-person perspective showing a needle deeply penetrating (pain condition) or a cotton-bud touching (touch control condition) the FDI in a model's right hand (figure 1c). A baseline video was also included showing a static right hand. In the touch and pain videos, the cotton-bud and needle made initial contact with the skin by 700 ms, with full cotton-bud depression and needle penetration by 1100 ms. All movement across all video stimuli ceased at 1100 ms. Each trial comprised one video followed by a 5-s blank screen. During each trial, a single TMS pulse was administered at one of three timepoints between 1100 ms and 1400 ms to prevent habituation. A total of 144 trials were presented randomly across four blocks, with a fully factorial combination of video type (pain, touch or static) and pulse timepoint repeated four times per block for a total of 36 trials per block. Participants were given a 2-min break between blocks.
Day 2: (ii) Imitation control task
After TMS, participants completed the imitation control task. During this task, participants were presented with the same finger movement videos used for the training on the previous day ([39] and figure 1b for further details). A total of 120 trials were presented randomly across two blocks, with a fully factorial combination of irrelevant stimulus movement (index or middle finger lift) and response (index or middle finger lift, instructed by a number cue of ‘1’ or ‘2’, respectively) repeated 15 times per block for a total of 60 trials per block, comprising 30 congruent and 30 incongruent trials. Response times were measured from the onset of the number cue and irrelevant finger movement. Trials with response times less than 150 ms or greater than 2000 ms ([50]; 0.2 ± 0.2% of trials) were removed prior to analysis.
Day 2: (iii) Control reaction time task
Participants then completed a simple response time task to assess whether self–other control training influenced processing speeds for the trained stimuli. Participants were presented with three pairs of images: (i) red triangle followed by blue square (non-social control); (ii) cotton-bud away from hand followed by cotton-bud pressing on FDI (touch) and (iii) needle away from hand followed by needle penetrating FDI (pain) (figure 1d) and were instructed to press the space bar as soon as the first image changed to the second image. To prevent habituation, four different time intervals (900, 1000, 1100 and 1200 ms) were used between the first and second images. A fully factorial combination of time interval and image pair type (non-social control, touch, pain) was repeated five times to a total of 60 trials. Mean response times were measured from the onset of the second image. Trials with response times less than 150 ms or greater than 2000 ms (4.9 ± 1.0% of trials) were removed prior to analysis.
(ii). Results
Corticospinal empathy
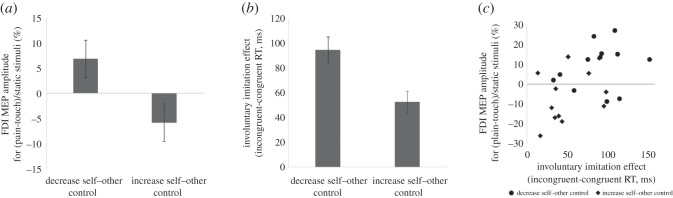
Corticospinal empathy was analysed using an independent-samples t-test comparing the two groups (increased self–other control and decreased self–other control). Normalized MEP size in the FDI target muscle for observation of pain, compared with observation of touch, was significantly lower in the increased self–other control group (−5.8 ± 3.8%) as compared to the decreased self–other control group (6.8 ± 3.7%, t23 = 2.387, p = 0.026, d = 0.956; figure 2a). This suggests that participants trained to increase self–other control demonstrated increased corticospinal empathy, as demonstrated by increased MEP inhibition when observing painful versus tactile stimulation, compared with those trained to decrease self–other control.
Figure 2.
Experiment 1: results of training to increase or decrease self–other control on (a) corticospinal empathic inhibition and (b) control of involuntary imitation. (c) Correlation between corticospinal empathic inhibition and control of involuntary imitation after training.
Imitation control
The imitation control effect was calculated by subtracting mean response time on congruent trials from that on trials requiring imitation control; therefore, higher values reflect a failure of self–other control. The increased self–other control group had a lower imitation control effect (53 ± 9 ms) than the decreased self–other control group (95 ± 11 ms, t23 = 3.030, p = 0.006, d = 1.219; figure 2b). This suggests that participants trained to increase self–other control demonstrated an increased ability to control involuntary imitation.
In order to establish whether training-related differences in empathy were related to the ability to control imitation, a correlation analysis was performed following removal of one multivariate outlier. Empathy was significantly correlated with the ability to control imitation (r24 = 0.488, p = 0.016; figure 2c), such that participants with increased corticospinal empathic inhibition were also better able to control the tendency to imitate others.
Control response time task
Electronic supplementary material, table S2 displays mean response times for the three stimulus types for each training group. A mixed ANOVA was conducted on the response times with stimulus type (non-social control, touch, pain) as within-subjects factor and group (decreased self–other control, increased self–other control) as between-subjects factor. There was no main effect of group (F1,23 = 2.167, p = 0.155), no main effect of stimulus type (F2,46 = 2.425, p = 0.100) and no group × stimulus type interaction (F2,46 = 0.063, p = 0.939) which suggests that training did not influence response times.
(iii). Discussion
The results of Experiment 1 suggest that training to control representations of the self and others in one socio-cognitive domain can transfer to another social domain. Participants trained to increase self–other control in the motor domain demonstrated increased corticospinal empathy, 24 h after training, compared with a group trained to decrease self–other control. In line with a previous study [38], participants trained to increase self–other control also demonstrated an increased ability to control involuntary imitation, and there was a moderate relationship between participants' scores across these two socio-cognitive domains.
It is possible that the altered corticospinal empathy following training to increase self–other control can be interpreted as an improved ability to withhold a motor response during pain observation (e.g. [51]). However, given that the control reaction time task did not demonstrate an effect of training on response times to pain stimuli, that speeded motor responses were required in both training conditions and that pain stimuli were not used during training, it seems unlikely that the effect of training is due to an improved ability to withhold a motor response to these stimuli. It is also possible that the use of MEPs to index corticospinal empathy increased the likelihood of finding an effect of self–other control training on empathy, because the training was administered in the motor domain and MEPs were measured in response to stimulation of primary motor cortex. However, the use of a measure combining touch, pain and static MEPs rules out the possibility that the effects of training are due solely to changes in corticospinal excitability caused by training. In addition, effects on corticospinal empathy were found 24 h after training, suggesting that they are unlikely to be due to immediate changes in corticospinal excitability as a result of the inhibitory demands of self–other control training. In order to rule out this possibility, however, Experiment 2 used a self-report measure of empathy to assess the effects of training.
Finally, it is possible that the apparent effects of self–other control training on corticospinal empathy in Experiment 1 were not due to the training itself, but instead were due to pre-existing differences between groups in corticospinal empathy due to random sampling error (although no pre-training differences were present in self-reported empathy; see Experiment 1 Participants section). Therefore, Experiment 2 measured the effects of training in terms of change from a pre-training baseline. This also allows assessment of whether the effects found in Experiment 1 result from an increase in empathy in the increased self–other control training group, or alternatively from a decrease in empathy in the other training group.
(b). Experiment 2
In contrast with Experiment 1, which used an implicit measure of empathy, Experiment 2 assessed whether self–other control training could alter an explicit, self-report measure of empathy. This enabled the assessment of whether the results found in Experiment 1 were specific to the particular measure used. Different training stimuli and actions were used to ensure that the results of Experiment 1 were not specific to one type of action. In addition, self-reported empathy was measured both before and after training, enabling assessment of which training type drives the effects of training.
(i). Methods
Participants
Forty-four right-handed participants (16 male) aged 18–35 years (mean 21.8) were recruited from the University of Surrey, screened for normal or corrected to normal vision, and randomly assigned to either the decreased self–other control or increased self–other control training group. Participants attended on two consecutive days.
Procedure
Participants completed the Questionnaire of Cognitive and Affective Empathy (QCAE; [52]) during the pre-screening stage of the experiment to enable pre- and post-training comparison of self-reported empathy. This questionnaire comprises 31 items (sample: ‘I am good at predicting how someone will feel’) to which participants must respond with one of four choices (strongly agree, slightly agree, slightly disagree and strongly disagree), across five subscales, which index both cognitive and affective empathy.
On Day 1, participants (i) completed an imitation control task and (ii) received either imitation training or imitation–inhibition training to decrease or increase self–other control, respectively. On Day 2, participants: (i) completed the imitation control task again and (ii) completed the QCAE again. Testing was carried out by experimenters who were blind to the hypothesized direction of training effects. Following testing, participants were debriefed. No participants reported awareness of the link between the Day 1 training and the Day 2 empathy questionnaire.
Day 1: (i) Imitation control task
Participants completed a task based on Heyes et al. [53] but using stimuli depicting a goal-directed button-pressing movement of the index or middle finger of a left hand (figure 1e; the electronic supplementary material).
Day 1: (ii) Training to decrease or increase self–other control
Training followed the same procedure as for Experiment 1, with the following changes. The stimulus and response movements were goal-directed button-press movements (figure 1f) as used in the imitation control task of Experiment 2. Participants in the decreased self–other control group were asked to press the ‘V’ key with their index finger when the index finger of the stimulus hand performed a button-press, and to press the ‘B’ key with their middle finger when the middle finger of the stimulus hand performed a button-press. Participants in the increased self–other control group were asked to press the ‘B’ key with their middle finger when the index finger of the stimulus hand performed a button-press, and to press the ‘V’ key with their index finger when the middle finger of the stimulus hand performed a button-press. A total of 432 trials were presented randomly across six blocks, with each stimulus movement (index or middle finger press) repeated 36 times per block. Response times were measured from the onset of the finger movement.
(ii). Results
A mixed ANOVA performed on the total QCAE scores with between-subjects factor of group (increased self–other control, decreased self–other control) and within-subjects factor of session (pre-training, post-training) revealed an interaction between group and session, F1,42 = 6.88, p = 0.012, 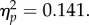 Post hoc tests confirmed that there was no difference between the groups at pre-test (increased self–other control: 86.32 ± 2.02; decreased self–other control: 88.77 ± 1.83, t42 = 0.902, p = 0.372) and that only the increased self–other control group demonstrated a significant change between the two sessions (increased self–other control, post-training: 89.86 ± 1.87; t21 = 2.524, p = 0.020, d = 0.37; decreased self–other control, post-training: 87.59 ± 1.73; t21 = 1.046, p = 0.307), which suggests that training to increase self–other control increased self-reported empathy, and supports the suggestion that the results of Experiment 1 were specifically due to the effect of the increased self–other control training on empathy.
Post hoc tests confirmed that there was no difference between the groups at pre-test (increased self–other control: 86.32 ± 2.02; decreased self–other control: 88.77 ± 1.83, t42 = 0.902, p = 0.372) and that only the increased self–other control group demonstrated a significant change between the two sessions (increased self–other control, post-training: 89.86 ± 1.87; t21 = 2.524, p = 0.020, d = 0.37; decreased self–other control, post-training: 87.59 ± 1.73; t21 = 1.046, p = 0.307), which suggests that training to increase self–other control increased self-reported empathy, and supports the suggestion that the results of Experiment 1 were specifically due to the effect of the increased self–other control training on empathy.
(iii). Discussion
Experiment 2 revealed that training to increase control of representations of the self and others in the motor domain increases an explicit, self-report, measure of empathy, compared with a group trained to decrease self–other control. As with Experiment 1, these effects were found 24 h after training. These results are supportive of the results found in Experiment 1: although the use of a different measure of empathy means that the two experiments cannot be directly compared, these results support the suggestion that the results of Experiment 1 were not an artefact of the measure used in that experiment, nor were they due to pre-existing differences between the training groups. These results are also consistent with the suggestion that the effects of both experiments are due to increased empathy in the group trained to increase self–other control, rather than decreased empathy in the group trained to decrease self–other control; however, this conclusion must remain tentative because of the use of different training stimuli and actions, and different measures of empathy across the two experiments.
(c). General discussion
The importance of self–other control for social interaction has previously been demonstrated for imitation and perspective-taking [38] but this is the first study to extend this finding into the domain of empathy.
Experiment 1 demonstrated that participants trained to increase self–other control showed increased empathic corticospinal responses when observing painful versus tactile stimulation applied to another person, compared with those trained to decrease self–other control. They also demonstrated an increased ability to control imitation, and a moderate relationship was found between scores across these two socio-cognitive domains. No effect of training was found on subjective ratings of the pain and touch videos (see the electronic supplementary material) or on response times to the stimuli, indicating that these effects were not due to increased attention to, or perceptual processing of, the pain stimuli, or increased ability to withhold a motor response. Experiment 2 used different training stimuli and movements, and an explicit rather than an implicit measure of empathy, but found consistent effects: participants trained to increase self–other control showed increased self-reported empathy, compared with those trained to decrease self–other control. Thus, self–other control training modulated both an objective, implicit measure and an explicit, self-report measure of empathy for at least 24 h after training occurred.
During self–other control training, participants must inhibit the motor representation activated by the sight of another's action and enforce their own motor representation. We hypothesize that this leads to an increased ability to control representations of the other and the self across multiple social domains. The finding that self–other control training enhanced corticospinal empathic responses to others' pain supports the contention that in order to empathize with others, it is necessary to be able to control one's own emotional state [16]. We hypothesize that suppressing their own non-pain state allowed participants better automatically to simulate the other's pain. Alternatively, increased self–other control may have improved participants' ability to identify the activated representation of a pain state as ‘other’, reducing their PD and thus making them more able to simulate the other's pain [41]. These data are consistent with the suggestion that increased levels of mirroring of others' tactile and pain sensations in mirror-touch synaesthesia are the result of reduced self–other control.
One question resulting from these findings is whether the effects of self–other control training are specific to this type of training, or whether instead any type of executive function training could produce similar effects on empathy. In their previous study, Santiesteban et al. [38] demonstrated that training to increase self–other control, but not training in more general cognitive inhibition, improved the ability to take another person's visual perspective. Thus, it appears likely that general inhibition training would not have similar effects on empathy, but this does remain a question for further research. More broadly, it will be important for future research to clarify whether self–other control is a specifically social process or a sub-type of a more domain-general process [54,55].
The current findings can be compared with those of a recent paper demonstrating that corticospinal excitability during the observation of painful stimuli applied to another's hand was increased when that hand imitated the participant's actions, compared with when it did not [56]. The authors suggested that being imitated by the hand gave the participant a sense of control over the hand and that this increased corticospinal excitability. The direction of our results is consistent with this finding, but two significant methodological differences suggest a different interpretation. In this study, participants had no sense of control over the hand during training because they responded to the movements of the hand, rather than vice versa; and the pain stimuli were applied to a hand that was markedly different in orientation and background from the hand to which they responded during training. Thus, our data suggest that de Coster et al.'s [56] results may instead be due to a general effect of being imitated on representations of the self and the other. It is important to note that their ‘exerting control’ condition consisted of participants who were not exerting self–other control as in our study, but instead were imitated by the hand on the screen. We suggest that this is more similar to our ‘decreased self–other control’ group: both of these groups should show more self–other overlap following training, thus being less able to control the affective response to the other's pain and hence showing higher corticospinal excitability, compared with the increased self–other control group.
Sensorimotor training similar to that used in the present experiments has also been used to demonstrate the effects of experience on ‘mirror’ responses to others' actions ([53,57]; see [6] for a review). Sensorimotor experience can build new associations between sensory and motor representations: for example, typical social experience often produces ‘mirror’ associations—that is, associations between sensory and motor representations corresponding to the same action. After such associations are formed, however, training to increase self–other control has two distinct effects on social cognition. It not only produces new ‘counter-mirror’ associations, but also improves the ability to control self- and other-relevant representations, by training the participant to inhibit the automatic activation of other-relevant representations (resulting from the presence of an initial mirror association). We suggest, therefore, that training to increase self–other control will only be effective where other-relevant representations are already associated with one's own representations of those attributes.
The current data are among the first to demonstrate that a short behavioural intervention in one socio-cognitive domain can modify social cognitive functioning in another domain (see also [38]), and the first to show such effects on empathy. The finding that self–other control training not only modulated corticospinal empathy and self-reported empathy but also increased the ability to control imitation suggests that the relationships between these different social cognitive domains are mediated by self–other control processes. Our data suggest, therefore, that although the control of imitation and self–other control in the affective domain may produce responses in adjacent brain areas [16], it is likely that these areas (the TPJ and supramarginal gyrus) perform the same computations on different inputs (and with different outputs) as a product of their distinct anatomical connectivity [58] (see [59] for a comparable example of two adjacent brain areas implementing the same computational process on different inputs as a function of each area's anatomical connectivity). However, it will be important for future research to follow-up the current results by testing whether the neural networks underlying self–other control training can be specifically isolated to TPJ or supramarginal gyrus, or whether in fact both areas are affected by this type of training.
The current results suggest that the control of neural representations of the self and other is an ability that is crucial for many types of social interaction, and also pave the way for the use of behavioural interventions to improve cognition across multiple social domains.
Supplementary Material
Acknowledgements
We thank C. Bowden, L. de la Fosse and H. Jones for their assistance in data collection for Experiment 2.
Ethics
For both experiments, written informed consent was obtained prior to taking part according to the Declaration of Helsinki and participants were paid a small honorarium for their time. Experimental procedures were approved by the University of Surrey Ethics Committee.
Data accessibility
The datasets supporting this article are available through the Surrey Research Insight research repository.
Authors' contributions
M.d.G. contributed to study design; data acquisition, analysis and interpretation; drafting and revising the article; final approval. G.B. contributed to study design; data analysis and interpretation; drafting and revising the article; final approval. M.J.B. contributed to data interpretation; drafting and revising the article; final approval. C.C. contributed to study design; data acquisition, analysis and interpretation; drafting and revising the article; final approval.
Competing interests
We have no competing interests.
Funding
This work was supported by the Economic and Social Research Council (ES/K00140X/1 to C.C.; ES/K00882X/1 to M.J.B.).
References
- 1.Spengler S, Bird G, Brass M. 2010. Hyperimitation of actions is related to reduced understanding of others’ minds in autism spectrum conditions. Biol. Psychiatry 68, 1148–1155. ( 10.1016/j.biopsych.2010.09.017) [DOI] [PubMed] [Google Scholar]
- 2.di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. 1992. Understanding motor events: a neurophysiological study. Exp. Brain Res. 91, 176–180. ( 10.1007/BF00230027) [DOI] [PubMed] [Google Scholar]
- 3.Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. 2000. Reading the mind in cartoons and stories: an fMRI study of 'theory of mind' in verbal and nonverbal tasks. Neuropsychologia 38, 11–21. ( 10.1016/S0028-3932(99)00053-6) [DOI] [PubMed] [Google Scholar]
- 4.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. 2004. Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162. ( 10.1126/science.1093535) [DOI] [PubMed] [Google Scholar]
- 5.Heyes CM, Bird G. 2007. Mirroring, association and the correspondence problem. In Sensorimotor foundations of higher cognition, attention and performance (eds Haggard P, Rossetti Y, Kawato M), pp. 481–482. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Cook R, Bird G, Catmur C, Press C, Heyes C. 2014. Mirror neurons: from origin to function. Behav. Brain Sci. 37, 177–192. ( 10.1017/S0140525X13000903) [DOI] [PubMed] [Google Scholar]
- 7.Brass M, Ruby P, Spengler S. 2009. Inhibition of imitative behaviour and social cognition. Phil. Trans. R. Soc. B 364, 2359–2367. ( 10.1098/rstb.2009.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keysar B, Barr DJ, Balin JA, Brauner JS. 2000. Taking perspective in conversation: the role of mutual knowledge in comprehension. Psychol. Sci. 11, 32–38. ( 10.1111/1467-9280.00211) [DOI] [PubMed] [Google Scholar]
- 9.Wimmer H, Perner J. 1983. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children's understanding of deception. Cognition 13, 103–128. ( 10.1016/0010-0277(83)90004-5) [DOI] [PubMed] [Google Scholar]
- 10.Decety J, Meyer M. 2008. From emotion resonance to empathic understanding: a social developmental neuroscience account. Dev. Psychopathol. 20, 1053–1080. ( 10.1017/S0954579408000503) [DOI] [PubMed] [Google Scholar]
- 11.Decety J, Lamm C. 2007. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist 13, 580–593. ( 10.1177/1073858407304654) [DOI] [PubMed] [Google Scholar]
- 12.Spengler S, von Cramon DY, Brass M. 2009. Control of shared representations relies on key processes involved in mental state attribution. Hum. Brain Mapp. 30, 3704–3718. ( 10.1002/hbm.20800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santiesteban I, Banissy MJ, Catmur C, Bird G. 2012. Enhancing social ability by stimulating right temporoparietal junction. Curr. Biol. 22, 2274–2277. ( 10.1016/j.cub.2012.10.018) [DOI] [PubMed] [Google Scholar]
- 14.Sowden S, Catmur C. 2015. The role of the right temporoparietal junction in the control of imitation. Cereb. Cortex 25, 1107–1113. ( 10.1093/cercor/bht306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young L, Camprodon J, Hauser M, Pascual-Leone A, Saxe R. 2010. Disruption of the right temporo-parietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgment. Proc. Natl Acad. Sci. USA 107, 6753–6758. ( 10.1073/pnas.0914826107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silani G, Lamm C, Ruff CC, Singer T. 2013. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J. Neurosci. 33, 15 466–15 476. ( 10.1523/JNEUROSCI.1488-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossi D, Marcone R, Cinquegrana T, Gallucci M. 2012. On the differential nature of induced and incidental echolalia in autism. J. Intellect. Disabil. Res. 57, 903–912. ( 10.1111/j.1365-2788.2012.01579.x) [DOI] [PubMed] [Google Scholar]
- 18.Russell J. 1997. Autism as an executive disorder. New York, NY: Oxford University Press. [Google Scholar]
- 19.Sowden S, Koehne S, Catmur C, Dziobek I, Bird G In press. Intact automatic imitation and typical spatial compatibility in autism spectrum disorder: challenging the Broken Mirror Theory. Autism Res. ( 10.1002/aur.1511) [DOI] [PubMed] [Google Scholar]
- 20.Happé F, Frith U. 1995. Theory of mind in autism. In Learning and cognition in autism (eds Schopler E, Mesibov GB), pp. 177–197. New York, NY: Springer. [Google Scholar]
- 21.Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, Suckling J, Baron-Cohen S, MRC AIMS Consortium. 2010. Atypical neural self-representation in autism. Brain 133, 611–624. ( 10.1093/brain/awp306) [DOI] [PubMed] [Google Scholar]
- 22.Lombardo MV, Chakrabarti B, Bullmore ET, Baron-Cohen S, MRC AIMS Consortium. 2011. Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage 56, 1832–1838. ( 10.1016/j.neuroimage.2011.02.067) [DOI] [PubMed] [Google Scholar]
- 23.Rogers K, Dziobek I, Hassenstab J, Wolf OT, Convit A. 2007. Who cares? Revisiting empathy in Asperger syndrome. J. Autism Dev. Disord. 37, 709–715. ( 10.1007/s10803-006-0197-8) [DOI] [PubMed] [Google Scholar]
- 24.Smith A. 2009. The empathy imbalance hypothesis of autism: a theoretical approach to cognitive and emotional empathy in autistic development. Psychol. Rec. 59, 489–510. [Google Scholar]
- 25.Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G. 2007. Empathy and judging other's pain: an fMRI study of alexithymia. Cereb. Cortex 17, 2223–2234. ( 10.1093/cercor/bhl130) [DOI] [PubMed] [Google Scholar]
- 26.Guttman H, Laporte L. 2002. Alexithymia, empathy, and psychological symptoms in a family context. Compr. Psychiatry 43, 448–455. ( 10.1053/comp.2002.35905) [DOI] [PubMed] [Google Scholar]
- 27.Guttman HA, Laporte L. 2000. Empathy in families of women with borderline personality disorder, anorexia nervosa, and a control group. Fam. Process 39, 345–358. ( 10.1111/j.1545-5300.2000.39306.x) [DOI] [PubMed] [Google Scholar]
- 28.Blakemore SJ, Bristow D, Bird G, Frith C, Ward J. 2005. Somatosensory activations during the observation of touch and a case of vision-touch synesthesia. Brain 128, 1571–1583. ( 10.1093/brain/awh500) [DOI] [PubMed] [Google Scholar]
- 29.Banissy MJ, Kadosh RC, Maus GW, Walsh V, Ward J. 2009. Prevalence, characteristics and a neurocognitive model of mirror-touch synaesthesia. Exp. Brain Res. 198, 261–272. ( 10.1007/s00221-009-1810-9) [DOI] [PubMed] [Google Scholar]
- 30.Holle H, Banissy M, Wright TD, Bowling N, Ward J. 2011. ‘That's not a real body’: identifying stimulus qualities that modulate synaesthetic experiences of touch. Conscious Cogn. 20, 720–726. ( 10.1016/j.concog.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 31.Aimola Davies AMA, White RC. 2013. A sensational illusion: vision-touch synaesthesia and the rubber hand paradigm. Cortex 49, 806–818. ( 10.1016/j.cortex.2012.01.007) [DOI] [PubMed] [Google Scholar]
- 32.Banissy M, Ward J. 2007. Mirror touch synaesthesia is linked with empathy. Nat. Neurosci. 10, 815–816. ( 10.1038/nn1926) [DOI] [PubMed] [Google Scholar]
- 33.Goller AI, Richards K, Novak S, Ward J. 2013. Mirror-touch synaesthesia in the phantom limbs of amputees. Cortex 49, 243–251. ( 10.1016/j.cortex.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 34.Banissy MJ, Garrido L, Kusnir F, Duchaine B, Walsh V, Ward J. 2011. Superior facial expression, but not identity recognition, in mirror-touch synaesthesia. J. Neurosci. 31, 1820–1824. ( 10.1523/JNEUROSCI.5759-09.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banissy MJ, Ward J. 2013. Mechanisms of self-other representations and vicarious experiences of touch in mirror-touch synesthesia. Front. Hum. Neurosci. 7, 112 ( 10.3389/fnhum.2013.00112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holle H, Banissy MJ, Ward J. 2013. Functional and structural brain correlates of mirror-touch synaesthesia. NeuroImage 83, 1041–1050. ( 10.1016/j.neuroimage.2013.07.073) [DOI] [PubMed] [Google Scholar]
- 37.Santiesteban I, Bird G, Tew O, Cioffi C, Banissy MJ. 2015. Mirror-touch synaesthesia: difficulties inhibiting the other. Cortex 71, 116–121. ( 10.1016/j.cortex.2015.06.019) [DOI] [PubMed] [Google Scholar]
- 38.Santiesteban I, White S, Cook J, Gilbert SJ, Heyes C, Bird G. 2012. Training social cognition: from imitation to Theory of Mind. Cognition 122, 228–235. ( 10.1016/j.cognition.2011.11.004) [DOI] [PubMed] [Google Scholar]
- 39.Brass M, Bekkering H, Wohlschlager A, Prinz W. 2000. Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain Cogn. 44, 124–143. ( 10.1006/brcg.2000.1225) [DOI] [PubMed] [Google Scholar]
- 40.Avenanti A, Bueti D, Galati G, Aglioti SM. 2005. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat. Neurosci. 8, 955–960. ( 10.1038/nn1481) [DOI] [PubMed] [Google Scholar]
- 41.Avenanti A, Minio-Paluello I, Bufalari I, Aglioti SM. 2009. The pain of a model in the personality of an onlooker: influence of state-reactivity and personality traits on embodied empathy for pain. NeuroImage 44, 275–283. ( 10.1016/j.neuroimage.2008.08.001) [DOI] [PubMed] [Google Scholar]
- 42.Avenanti A, Sirigu A, Aglioti SM. 2010. Racial bias reduces empathic sensorimotor resonance with other-race pain. Curr. Biol. 20, 1018–1022. ( 10.1016/j.cub.2010.03.071) [DOI] [PubMed] [Google Scholar]
- 43.Minio-Paluello I, Baron-Cohen S, Avenanti A, Walsh V, Aglioti SM. 2009. Absence of embodied empathy during pain observation in Asperger syndrome. Biol. Psychiatry 65, 55–62. ( 10.1016/j.biopsych.2008.08.006) [DOI] [PubMed] [Google Scholar]
- 44.Urban PP, Solinski M, Best C, Rolke R, Hopf HC, Dieterich M. 2004. Different short-term modulation of cortical motor output to distal and proximal upper-limb muscles during painful sensory nerve stimulation. Muscle Nerve 29, 663–669. ( 10.1002/mus.20011) [DOI] [PubMed] [Google Scholar]
- 45.Borgomaneri S, Gazzola V, Avenanti A. 2014. Temporal dynamics of motor cortex excitability during perception of natural emotional scenes. Soc. Cogn. Affect Neurosci. 9, 1451–1457. ( 10.1093/scan/nst139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis MH. 1980. A multidimensional approach to individual differences in empathy. JSAS Catalog Selected Doc. Psychol. 10, 85. [Google Scholar]
- 47.Davis MH. 1983. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 44, 113–126. ( 10.1037/0022-3514.44.1.113) [DOI] [Google Scholar]
- 48.Catmur C, Heyes C. 2011. Time course analyses confirm independence of imitative and spatial compatibility. J. Exp. Psychol. Hum. Percept. Perform. 37, 409–421. ( 10.1037/a0019325) [DOI] [PubMed] [Google Scholar]
- 49.Rossini PM, et al. 1994. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 91, 79–92. ( 10.1016/0013-4694(94)90029-9) [DOI] [PubMed] [Google Scholar]
- 50.Cook J, Bird G. 2011. Social attitudes differentially modulate imitation in adolescents and adults. Exp. Brain Res. 211, 601–612. ( 10.1007/s00221-011-2584-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrison I, Poliakoff E, Gordon L, Downing P. 2007. Response-specific effects of pain observation on motor behavior. Cognition 104, 407–416. ( 10.1016/j.cognition.2006.07.006) [DOI] [PubMed] [Google Scholar]
- 52.Reniers RL, Corcoran R, Drake R, Shryane NM, Völlm BA. 2011. The QCAE: a Questionnaire of Cognitive and Affective Empathy. J. Pers. Assess. 93, 84–95. ( 10.1080/00223891.2010.528484) [DOI] [PubMed] [Google Scholar]
- 53.Heyes C, Bird G, Johnson H, Haggard P. 2005. Experience modulates automatic imitation. Cogn. Brain Res. 22, 233–240. ( 10.1016/j.cogbrainres.2004.09.009) [DOI] [PubMed] [Google Scholar]
- 54.Cook JL. 2014. Task-relevance dependent gradients in medial prefrontal and temporoparietal cortices suggest solutions to paradoxes concerning self/other control. Neurosci. Biobehav. Rev. 42, 298–302. ( 10.1016/j.neubiorev.2014.02.007) [DOI] [PubMed] [Google Scholar]
- 55.Heyes C, Catmur C. 2015. A task control theory of mirror-touch synaesthesia. Cogn. Neurosci. 6, 141–142. ( 10.1080/17588928.2015.1057485) [DOI] [PubMed] [Google Scholar]
- 56.de Coster L, Andres M, Brass M. 2014. Effects of being imitated on motor responses evoked by pain observation: exerting control determines action tendencies when perceiving pain in others. J. Neurosci. 34, 6952–6957. ( 10.1523/JNEUROSCI.5044-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Press C, Catmur C, Cook R, Widmann H, Heyes C, Bird G. 2012. fMRI evidence of ‘mirror’ responses to geometric shapes. PLoS ONE 7, e51934 ( 10.1371/journal.pone.0051934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steinbeis N, Bernhardt BC, Singer T. 2014. Age-related differences in function and structure of rSMG and reduced functional connectivity with DLPFC explains heightened emotional egocentricity bias in childhood. Soc. Cogn. Affect. Neurosci. 10, 302–310. ( 10.1093/scan/nsu057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Behrens TE, Hunt LT, Woolrich MW, Rushworth MF. 2008. Associative learning of social value. Nature 456, 245–249. ( 10.1038/nature07538) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available through the Surrey Research Insight research repository.