Table 2.
Previous reports on the copper-catalyzed Huisgen cycloaddition to bistriazoles with spacers.
| Cu source | Solvent | Spacer | Alkyne | Application |
| Cu(OAc)2, sodium ascorbate [69] |
H2O/t-BuOH | 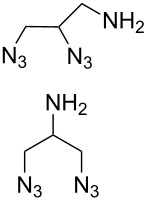 |
 |
Complexation |
| CuI [70–71] |
THF/H2O | 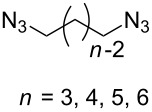 |
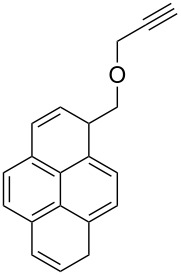 |
Chemical sensor |
| CuSO4·5H2O, sodium ascorbate [72] |
DMF/H2O | 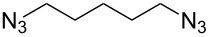 |
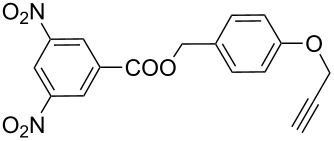 |
Supermolecular chemistry |
| CuSO4·5H2O, sodium ascorbate [73] |
DMF | 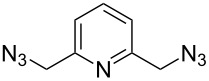 |
 |
Cytotoxic activity |
| [74] | CHCl3 | 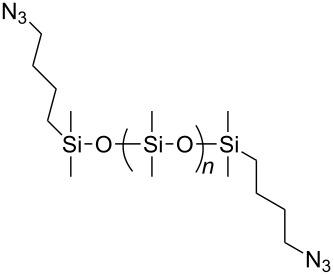 |
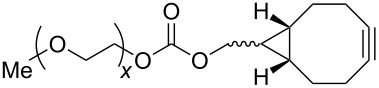 |
Polymer chemistry |
| CuSO4·5H2O, sodium ascorbate [75] |
THF/H2O | 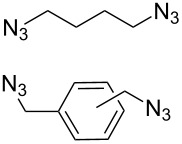 |
 |
– |
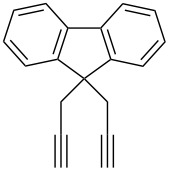
| CuSO4·5H2O, sodium ascorbate [76] |
H2O/t-BuOH |  |
 |
DNA binding |
| CuSO4·5H2O, sodium ascorbate [77] |
THF/H2O |  |
 |
Receptor |
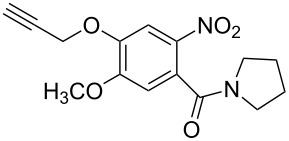
| CuSO4·5H2O, sodium ascorbate [78] |
DMF/H2O |  |
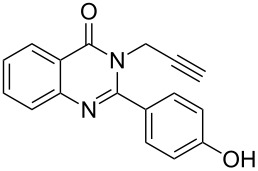 |
Molecular recognition |
| Cu(OAc)2/Cu [79] |
DMSO | 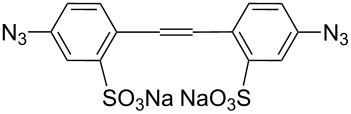 |
 |
Fluorescence brightening agents |
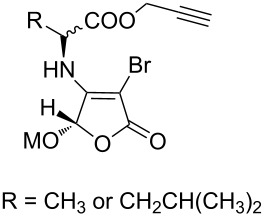
| CuSO4/Cu [80] |
EtOH |  |
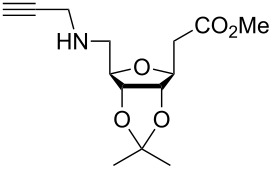 |
Chemical sensor |
| CuI [81] |
CH2Cl2/MeOH | 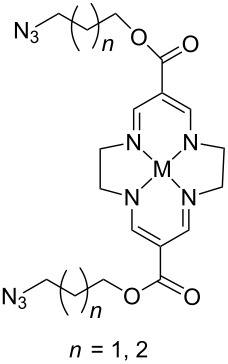 |
 |
Electroactive receptor |
| Cu(OAc)2 sodium ascorbate [82] |
t-BuOH | 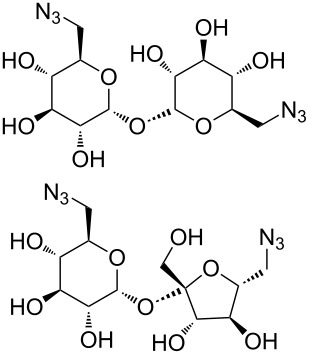 |
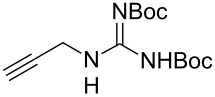 |
Biological activity |
