Table 2.
Preparation of 1-substituted 1H-1,2,3,4-tetrazoles in the presence of Cu NPs/bentonite by reaction between sodium azide, primary amines and triethyl orthoformate at 120 °C.a
| Entry | Substrate | Product | Yield [%]b |
| 1 | 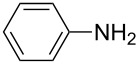 |
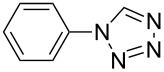 |
93 |
| 2 | 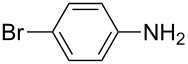 |
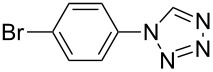 |
85 |
| 3 | 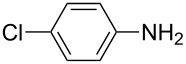 |
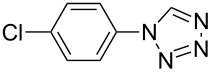 |
94 |
| 4 | 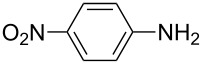 |
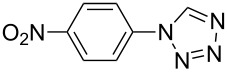 |
87 |
| 5 | 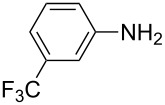 |
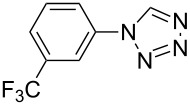 |
83 |
| 6 | 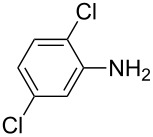 |
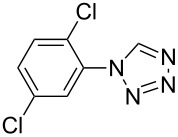 |
85 |
| 7 | 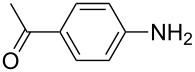 |
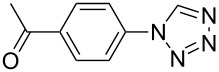 |
87 |
| 8 | 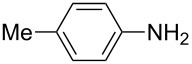 |
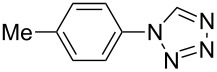 |
87 |
| 9 | 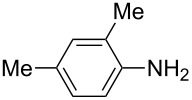 |
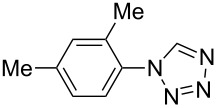 |
88 |
| 10 | 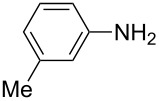 |
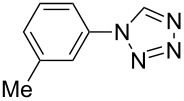 |
92 |
| 11 | 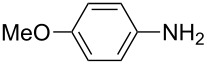 |
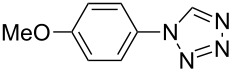 |
86 |
| 12 | 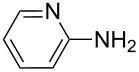 |
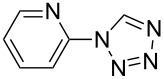 |
83 |
aReaction conditions: Amine (2.0 mmol), NaN3 (2 mmol), triethyl orthoformate (2.4 mmol), Cu NPs/bentonite (0.05 g) at 120 °C, 3 h. bIsolated yield.
