Table 1.
Asymmetric alkylation to 3-chloro-3,6-dihydro-2H-pyran (2a)a.
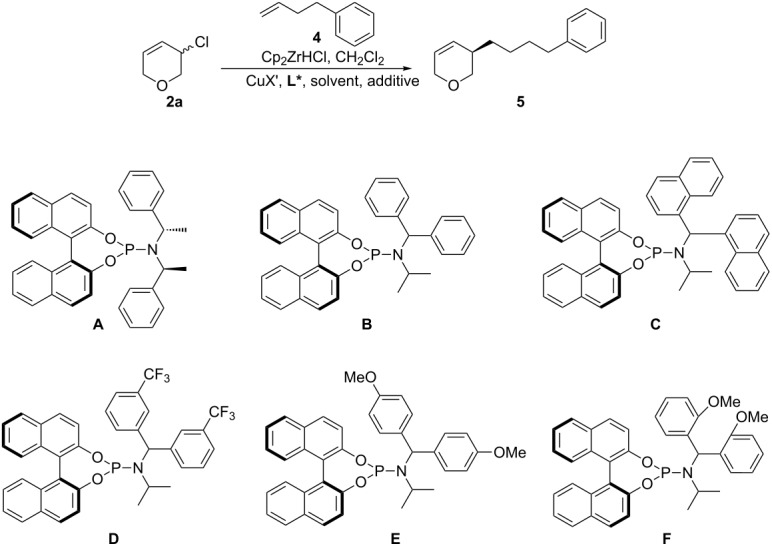 | |||||
| Entry | Copper | L* | Solvent | Additive | eeb |
| 1 | CuI | A | CHCl3 | NP | |
| 2 | CuClO4 | A | CH2Cl2 | 68% | |
| 3 | CuClO4 | B | CH2Cl2 | 70% | |
| 4 | CuOTf | B | CH2Cl2 | 64% | |
| 5 | CuNTf2 | B | CH2Cl2 | 52% | |
| 6 | CuTC | B | CH2Cl2 | 12% | |
| 7 | CuSbF6 | B | CH2Cl2 | NP | |
| 8 | CuClO4 | B | Et2O | 55% | |
| 9 | CuClO4 | B | Me-THF | 38% | |
| 10 | CuClO4 | B | CHCl3 | 67% | |
| 11 | CuClO4 | C | CH2Cl2 | 53% | |
| 12 | CuClO4 | D | CH2Cl2 | 36% | |
| 13 | CuClO4 | E | CH2Cl2 | 12% | |
| 14 | CuClO4 | B | CH2Cl2 | TMSCl | 73% |
| 15 | CuClO4 | B | CH2Cl2 | Si(OEt)4 | 63% |
| 16 | CuClO4 | B | CH2Cl2 | Ti(OiPr)4 | 25% |
| 17 | CuClO4 | B | CH2Cl2 | AlCl3 | 15% |
| 18 | CuClO4 | B | CH2Cl2 | B(OiPr)3 | 80% |
| 19 | CuClO4 | C | CH2Cl2 | B(OiPr)3 | 78% |
| 20 | CuClO4 | E | CH2Cl2 | B(OiPr)3 | 47% |
| 21 | CuClO4 | F | CH2Cl2 | B(OiPr)3 | 83% |
aConditions: 4-phenyl-1-butene (2.5 equiv), Cp2ZrHCl (2.0 equiv), 2a (1.0 equiv), CuL* as specified (0.1 equiv), additive as specified (1.0 equiv), in specified solvent (2.0 mL), room temperature. bee determined by HPLC. NP = no product. For more information on procedures see Supporting Information File 1.
