Table 1. Synthesis of N-Boc-Protected Azobenzene–Alanine Analogues.
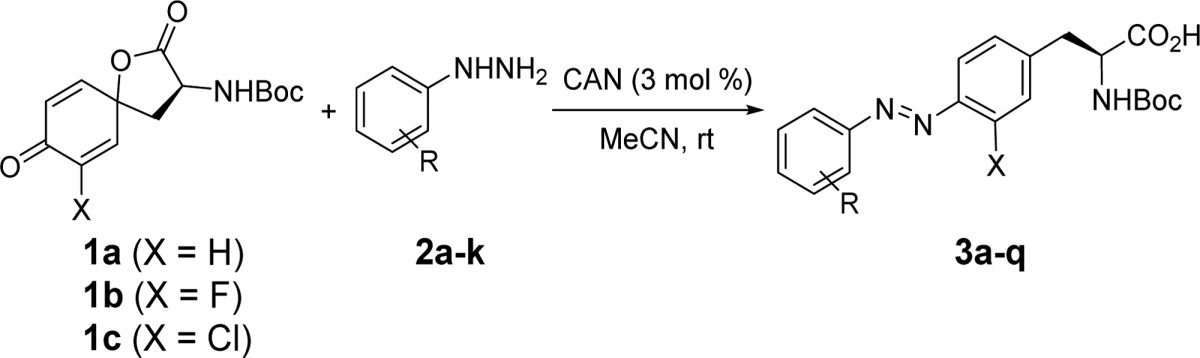
| entry | X | R | product | yielda (%) |
|---|---|---|---|---|
| 1 | H | 4-Me (2a) | 3a | 73 |
| 2 | H | 2-OMe (2b) | 3b | 72 |
| 3 | H | 4-CN (2c) | 3c | 72 |
| 4 | H | 3-CN (2d) | 3d | 85 |
| 5 | H | 3-CH=CH2 (2e) | 3e | 68 |
| 6 | H | 3-C≡CH (2f) | 3f | 83b |
| 7 | H | 2,6-F2 (2g) | 3g | 95 |
| 8 | H | 2,4,6-F3(2h) | 3h | 91 |
| 9 | H | 2,6-F2-4-CONH2 (2i) | 3i | 94c |
| 10 | H | 2,6-F2-4-I (2j) | 3j | 82d,e |
| 11 | H | 2,3,4,5,6-F5 (2k) | 3k | 89 |
| 12 | F | 2,6-F2 (2g) | 3l | 72 |
| 13 | F | 2,4,6-F3 (2h) | 3m | 72 |
| 14 | F | 2,3,4,5,6-F5 (2k) | 3n | 60 |
| 15 | Cl | 2,6-F2 (2g) | 3o | 68e,f |
| 16 | Cl | 2,4,6-F3 (2h) | 3p | 67f,g |
| 17 | Cl | 2,3,4,5,6-F5 (2k) | 3q | 63 |
Isolated yields were reported. Unless noted otherwise, reactions were carried out by stirring a solution of spirolactone (1a–c) with 1.1 equiv of the substituted phenylhydrazine in MeCN at room temperature for 12–17 h.
24 h reaction time.
MeOH/MeCN (1:4) was used as solvent.
1.5 equiv of the substituted phenylhydrazine was used.
72 h reaction time.
2.5 equiv of the substituted phenylhydrazine was used.
48 h reaction time.
