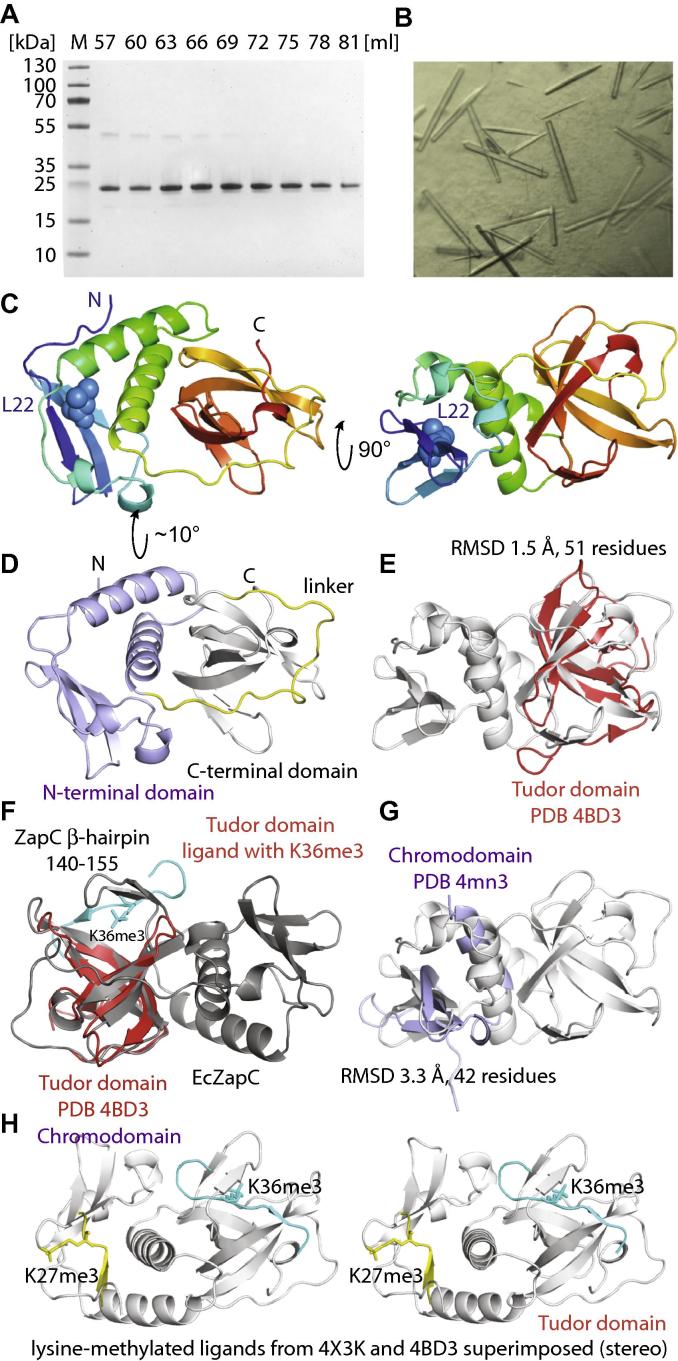
Fig. 1.
(A) Coomassie-stained SDS–PAGE gel showing fractions after size exclusion chromatography of E. coli SeMet ZapC. The first two fractions correspond to the void volume of the column used (see Section 2). (B) Typical crystals of E. coli SeMet ZapC after optimisation as used in this study. The needles were typically less than 20 μm in diameter, leading to weak diffraction and strong decay in the X-ray beam during data collection. Several wedges were collected from three crystals while translating along the long axis of the crystals and merged for structure determination. (C) Ribbon plot (PyMOL, Schrödinger) outlining the crystal structure of ZapC at 2.9 Å resolution. The structure is shown in rainbow colours from the N-terminus in blue to the C-terminus in red. Amino acid L22 is indicated by spheres [9]. (D) ZapC contains two fairly separate domains that are connected via a long linker, shown in yellow. The N-terminal domain is shown in light blue, the C-terminal domain in grey. (E) The C-terminal domain of ZapC is related to Tudor domains. Many such related domains come up in database structural similarity searches (DALI). A superposition between PDB 4BD3 (PHD finger protein 19, red, RMSD 1.5 Å over 51 Cα) [24] and ZapC’s C-terminal Tudor domain is shown. (F) Tudor domains are known to preferentially bind to peptides containing methylated arginines and lysines. The superposition in E is repeated here with the methylated (K36me3) histone tail peptide ligand shown in cyan. It is clear that the same binding pocket in ZapC is occluded by a small beta-hairpin comprising residues 141–154. (G) The N-terminal domain of ZapC is distantly related to chromo domains. Again, many such domains come up in database searches (PDBe FOLD). A superposition between PDB 4MN3 (CBX7, chromobox homologue 7, light blue, RMSD 3.3 Å over 42 Cα) [28] and ZapC’s chromo domain is shown. (H) Stereo plot showing both superpositions (from panels E, F; and G) with their lysine-methylated peptide ligands, only (yellow: chromo domain, cyan: Tudor domain). As for the Tudor domain, the canonical peptide pocket in ZapC’s chromo domain is probably occluded by a different orientation of the first strand and a small helical segment between residues 44 and 50.