Abstract
Vaccination is a useful option to control infection with porcine reproductive and respiratory syndrome virus (PRRSV), and several modified live-PRRSV vaccines have been developed. These vaccines have shown some efficacy in reducing the incidence and severity of clinical disease as well as the duration of viremia and virus shedding but have failed to provide sterilizing immunity. The efficacy of modified live-virus (MLV) vaccines is greater against a homologous strain compared with heterologous PRRSV strains. The objective of this study was to evaluate the efficacy of Fostera PRRS MLV vaccine in protecting against challenge with a heterologous field strain widely circulating in the swine herds of eastern Canada. Forty-six piglets were divided into 4 groups: nonvaccinated-nonchallenged; nonvaccinated-challenged; vaccinated-challenged; and vaccinated-nonchallenged. The animals were vaccinated at 23 d of age with Fostera PRRS and challenged 23 d later with a heterologous field strain of PRRSV (FMV12-1425619). Overall, the vaccine showed some beneficial effects in the challenged animals by reducing the severity of clinical signs and the viral load. A significant difference between nonvaccinated and vaccinated animals was detected for some parameters starting 11 to 13 d after challenge, which suggested that the cell-mediated immune response or other delayed responses could be more important than pre-existing PRRSV antibodies in vaccinated animals within the context of protection against heterologous strains.
Résumé
La vaccination est une option utile pour limiter l’infection par le virus du syndrome reproducteur et respiratoire porcin (VSRRP), et plusieurs vaccins VSRRP vivants modifiés ont été développés. Ces vaccins ont démontré une certaine efficacité à réduire l’incidence et la sévérité de la maladie clinique ainsi que la durée de la virémie et de l’excrétion virale mais ont failli à produire une immunité stérilisante. L’efficacité des vaccins vivants modifiés (VVM) est supérieure contre une souche homologue comparativement à des souches hétérologues de VSRRP. L’objectif de la présente étude était d’évaluer l’efficacité du vaccin Fostera, un VVM contre le VSSRP, à protéger contre une infection défi avec une souche de terrain hétérologue circulant librement dans les troupeaux porcins de l’est du Canada. Quarante-six porcelet ont été répartis en quatre groupes : non vaccinés-non infectés; non vaccinés-infectés; vaccinés-infectés; et vaccinés-non infectés. Les animaux ont été vaccinés à 23 jours d’âge avec Fostera SRRP et infectés 23 jours plus tard avec une souche de terrain hétérologue du VSRRP (FMV12-1425619). De manière générale, le vaccin a démontré quelques effets bénéfiques chez les animaux infectés en réduisant la sévérité des signes cliniques et la charge virale. Une différence significative entre les animaux non vaccinés et ceux vaccinés a été détectée pour quelques paramètres et débutant 11 à 13 j suite à l’infection, ce qui suggère que la réponse de l’immunité à médiation cellulaire ou d’autres réponses retardées pourraient être plus importantes que la présence d’anticorps anti-SRRP existants chez des animaux vaccinés dans le contexte d’une protection contre des souches hétérologues.
(Traduit par Docteur Serge Messier)
Introduction
Porcine reproductive and respiratory syndrome (PRRS) represents one of the most economically important viral diseases in the North American swine industry, causing losses estimated at 664 million US$ annually (1). The PRRS virus (PRRSV) is responsible for reproductive failure, characterized by late-term abortion and an increased incidence of stillbirth, prematurity, and/or weakness of the piglets. The virus is also responsible for increased rates of illness and death in growing and finishing pigs as a result of severe respiratory disease and poor growth performance (2,3).
The etiologic agent is an enveloped, single-strand, positive-sense RNA virus belonging to the Arteriviridae family, which includes lactate dehydrogenase-elevating virus of mice, simian hemorrhagic fever virus, and equine arteritis virus (4). The PRRSV RNA genome, of about 15 kb, is composed of at least 10 open reading frames (ORFs), which code for at least 7 structural proteins and 14 nonstructural proteins (5). As with many RNA viruses, the genome heterogeneity of PRRSV is the main hurdle to effective prevention and control of PRRS through vaccination (6). Strains of PRRSV have been classified into 2 main genotypes: genotype I (previously named European) and genotype II (previously named North American) (7). Genotype II strains circulating in North America can be classified into several subgenotypes (7–10). Interestingly, several of the subgenotype II strains circulating in the United States have not yet been reported in Canada (8–10), which suggests that some subgenotypes are geographically restricted. The 2 main genotypes are between 50% and 60% homologous in viral genomic nucleotides and are normally not cross-neutralized by antibodies raised against each other even though some level of cross-reactivity has been reported (11,12). Moreover, genetic and antigenic diversity exists within each genotype and negatively affects cross-protection among different viruses (13–15).
Vaccination is an important tool for controlling PRRSV infection. Many PRRSV vaccines have been developed, including products that contain live virus derived from cell-culture attenuation of virulent field isolates, inactivated preparations of attenuated PRRSV strains, inactivated preparations of virulent isolates expanded by in vitro cell culture for use as an autogenous vaccine, inactivated preparations of multiple virulent isolates enriched with viral antigens, and subunit vaccines expressing selected viral proteins (16). Modified live (or attenuated)-PRRSV vaccines have been widely used and have shown some efficacy in reducing the incidence and severity of clinical disease as well as the duration of viremia and virus shedding but have failed to provide complete sterilizing immunity (6). However, the efficacy of MLV vaccines is greater for homologous strains and can decline dramatically for genetically unrelated heterologous PRRSV strains. Use of the new MLV vaccine Fostera PRRS has been approved in Canada and the United States. This vaccine has been shown to reduce the levels of viremia and nasal shedding as well as the severity of PRRSV-induced lesions after experimental infection with a Korean heterologous strain (17). Unfortunately, most of the wild-type strains circulating in Canada are not from the same lineage as the Fostera PRRS vaccine strain. Therefore, the objective of this study was to evaluate the efficacy of Fostera PRRS in protecting against challenge with a heterologous virulent field strain widely circulating in the swine herds of eastern Canada.
Materials and methods
Animals
The procedures for animal care followed the guidelines of the Canadian Council on Animal Care (18), and the protocol was approved by the Institutional Animal Care Committee (Protocol 12-Rech-1669). Forty-six 16-day-old conventional piglets were obtained from a single farm with a common genetic and health background. The farm was negative for PRRSV, Mycoplasma hyopneumoniae, and swine influenzaviruses and had not been reported as having cases of postweaning multisystemic wasting syndrome related to porcine circovirus type 2 infection. The animals were randomly divided into 4 homogeneous groups: nonvaccinated-nonchallenged (n = 7); nonvaccinated-challenged (n = 15); vaccinated-challenged (n = 15); and vaccinated-nonchallenged (n = 9). The groups were housed in separate rooms with access to feed and water ad libitum.
Vaccination
After a 1-week acclimation period, the 23-day-old piglets were vaccinated intramuscularly (IM) with Fostera PRRS vaccine (lot A282040A) as recommended by the manufacturer (Zoetis Canada, Kirkland, Quebec). A placebo, phosphate-buffered saline (PBS), was given IM to the nonvaccinated piglets. The animals were weighed the day before inoculation to ensure that the experimental groups were homogeneous. No significant difference in body weight (P > 0.05) was found between the groups by one-way analysis of variance (ANOVA) with the parametric Tukey test (data not shown).
Challenge
Blood from pig farms experiencing an acute outbreak of PRRS was collected and the ORF5 gene of PRRSV sequenced to select a virulent heterologous strain by ORF5 phylogenetic analysis. The selected strain, FMV12-1425619 (accession no. KJ888950; GenBank, National Center for Biotechnology Information, Bethesda, Maryland, USA), was classified within a lineage-1 cluster of type II genotype frequently found in Quebec over the previous 2 y and often associated with clinical signs (unpublished data). Similarities in the PRRSV ORF5 amino acids between the selected field and vaccine strains were analyzed by means of the SIM alignment tool for protein sequences on the bioinformatics resource portal ExPASy (http://web.expasy.org/sim/). Several attempts to isolate the virus in MARC-145, SJPL, and PAM cells have failed. Thus, the viral inoculum used to challenge the animals was a lung tissue homogenate obtained from a piglet infected with 3 mL of PRRSV FMV12-1425619-positive serum. The median tissue culture infective dose (TCID50) of PRRSV in the homogenate was determined to be 1.5 × 104/mL by a previously described reverse-transcription quantitative polymerase chain reaction (RT-qPCR) method (19). A pilot study with 4 piglets confirmed the capacity of the challenge strain to induce PRRSV-specific clinical signs, viremia, and lung lesions in inoculated animals (data not shown). The homogenate was negative by PCR for bacteria (with a 16S gene PCR diagnostic assay), swine influenza virus, porcine parvovirus, and porcine circovirus type 2. The piglets in the nonvaccinated-challenged and the vaccinated-challenged groups were challenged 23 d after PBS or vaccine inoculation with 1 mL of the homogenate IM and 1 mL in each nostril. The other 2 groups were inoculated with PBS.
Monitoring and blood sampling
During the challenge period (28 d), body weight and body temperature were measured daily. The average daily gain (ADG) was calculated over different periods: i) from time of vaccination to day of challenge (postvaccination period); ii) during the first 13 d after challenge (day 0 to day 13); iii) during the first 27 d after challenge (postchallenge period); and iv) during the entire period of the experiment (postvaccination and postchallenge periods). The ADG was calculated by subtracting the initial body weight from the final body weight and dividing by the number of days for the different periods. Also, a growth rate was calculated to take into account the weight of the animals on challenge day since the weights of the experimental groups were not uniform on that day. This rate was calculated by dividing the weight gain over the period by the initial weight at the beginning of the period. Fever was defined as a rectal temperature higher than 40°C for 2 consecutive days. Clinical signs were scored daily according to the system presented in Table I. Blood samples were collected on days −3, 3, 7, 10, 13 to 14, 21, and 27 to 28 after challenge to determine the level of viremia by RT-qPCR. Nasal swabs were collected on days 7, 13, 21, and 27 after challenge to test for the presence of PRRSV RNA by real-time qPCR. At 14 d after challenge (i.e., 37 d after vaccination), 3, 4, 7, and 9 pigs were euthanized in the nonvaccinated-nonchallenged, vaccinated- nonchallenged, vaccinated-challenged, and nonvaccinated-challenged groups, respectively. The remaining animals were euthanized 28 d after challenge (i.e., 51 d after vaccination).
Table I.
System for scoring clinical signs of infection by porcine reproductive and respiratory syndrome virus
| Sign | Score |
|---|---|
| Sneezing | 1 |
| Nonproductive cough | |
| Light | 1 |
| Moderate | 2 |
| Severe | 3 |
| Productive cough | |
| Light | 2 |
| Moderate | 3 |
| Severe | 4 |
| Behavior | |
| Normal | 0 |
| Lethargic | 2 |
| Stimulus needed to take normal position after recumbency | 3 |
| Prolonged recumbency | 6 |
Euthanasia is required when the total score is ≥ 6 according to the Animal Care Ethic Committee.
Scoring of lung lesions
Macroscopic lung lesions were scored as previously described (20). The apex of the cranial lung lobes, intermediate dorsal sections of both right and left diaphragmatic lung lobes, and tracheobronchial lymph nodes were collected from each animal and fixed in 10% neutral buffered formalin for evaluation of specific microscopic lesions. Subsamples of those tissues were stored at −20°C until tested by RT-qPCR to determine viral load. Histopathological lesions were scored for severity of interstitial pneumonia as follows: 0 — normal, 1 — mild, 2 — moderate, 3 — important, and 4 — severe with alveolar disappearance. The presence of leukocytes, serum, or necrotic debris in alveolar exudate was scored as follows: 0 — normal, 0.5 — rare, 1 — mild, 2 — moderate, 3 — important, and 4 — severe. Finally, lymphoid follicular hyperplasia was scored as follows: 0 — normal, 1 — mild, 2 — moderate, and 3 — severe.
Quantification of PRRSV
Viremia level and viral load in nasal swabs and tissues were determined by RT-qPCR assay as previously described (19). Briefly, with use of the QIAamp Viral RNA kit (Qiagen, Mississauga, Ontario) viral RNA was isolated from serum samples and tissues according to the manufacturer’s instructions. A commercial PRRSV RT-qPCR diagnostic kit (NextGen, Tetracore, Rockville, Maryland, USA) was used for PRRSV quantification as recommended by the manufacturer. The quantity was determined by comparing the sample results with a standard curve based on the amount of serially diluted PRRSV IAF-Klop strain, which was produced in MARC-145 cells and subsequently titrated as the TCID50 of virus particles per milliliter of the MARC-145-infected cell culture supernatant. The RT-qPCR results were expressed in TCID50 per milliliter of serum or per gram of tissue.
Serum antibodies specific for PRRSV
Serum samples were tested with HerdChek PRRS X3 diagnostic enzyme-linked immunosorbent assay (ELISA) kits (IDEXX Laboratories, Westbrook, Maine, USA) according to the manufacturer’s instructions. The serum was diluted 1/40 in diluents supplied by the manufacturer. A sample-to-positive (S:P) ratio equal to or greater than 0.4 was considered positive.
Statistical analyses
All statistical analyses were done with GraphPad Prism software, version 5.03 (GraphPad Software, San Diego, California, USA). Parametric data (growth rate, rectal temperature, clinical-signs score, and virus titer) were analyzed by two-way ANOVA for repeated measures with the Bonferroni multiple-comparison test. The ADG was analyzed by a one-way ANOVA with the Tukey multiple- comparison test. Nonparametric data (antibody S:P ratios, lung-lesions scores) were analyzed by one-way ANOVA with the Kruskal–Wallis test. In some instances, data for vaccinated- challenged versus nonvaccinated-challenged animals were compared by applying Student’s unpaired t-test. A P-value < 0.05 was considered to reflect a statistically significant difference.
Results
Amino acid homology between the Fostera PRRS vaccine strain and the challenge strain, as determined by ORF5 genomic analysis, was 86% (Figure 1).
Figure 1.
Results of genomic analysis of similarities in the amino acid sequence of open reading frame 5 between a field strain of porcine reproductive and respiratory syndrome virus (PRRSV) (FMV12-1425619) and the vaccine strain used in this study (Fostera PRRS) by means of the SIM alignment tool for protein sequences on the bioinformatics resource portal ExPASy (http://web.expasy.org/sim/).
Animal exclusions
All the data related to the 4 animals described below were removed from the study. No histopathological abnormalities were found within the examined tissues other than lung.
One animal in the vaccinated-challenged group died 8 d after vaccination. No macroscopic or histopathological findings other than changes compatible with postmortem modifications were observed at necropsy. The animal’s lungs were PRRSV-positive by PCR.
Another animal in the vaccinated-challenged group was euthanized 8 d after challenge because of excessive weight loss (> 10% of total weight over 2 d). This animal had shown whitish nasal discharges before challenge. Consequently, nasal swabs were collected from several animals in each experimental group and tested for the presence of respiratory pathogens (such as Influenza A virus, porcine circovirus type 2, PRRSV, M. hyopneumoniae, M. hyorhinis, and Streptococcus suis). Analyses showed that the euthanized animal was PCR-positive for PRRSV, as expected, and for M. hyorhinis and S. suis; however, these 2 bacteria were also detected in animals in all the other experimental groups (data not shown). Macroscopically, interstitial pneumonia covering several regions of the lung tissue was observed, along with a large emphysematous lesion in the right diaphragmatic lobe. This type of lesion has no direct cause-and-effect relationship with PRRSV infection and is a rare finding in swine. Overall, the macroscopic lesions were estimated to affect 48% of the lung tissue, but after removal of the emphysematous lesion from consideration the lung-lesion score of this pig was established as 36%. Microscopic lung lesions, such as interstitial pneumonia and hyperplasia of the bronchus-associated lymphoid tissue, were related to PRRSV infection. Nonetheless, the data for this pig were removed from all analyses, mainly because of the unexpected and marked emphysematous lesion.
At 20 d after challenge 1 pig in the vaccinated-nonchallenged group died suddenly during blood collection. Although a small hemorrhage was observed at the site of blood collection, no other macroscopic lesions were found at necropsy. No histopathological findings related to PRRSV infection were found. Interestingly, several multifocal hemorrhages were found in the lung tissue, but no direct link could be made with the animal’s sudden death.
At 9 d after challenge 1 pig in the nonvaccinated-nonchallenged group died suddenly. On arrival for the study this animal had been cachectic, had locomotor problems, and weighed less than the other animals in the same group but was kept in the study. No macroscopic lung lesions and no histopathological findings related to PRRSV infection were found. All PRRSV PCR assay results were negative for this animal.
Antibody response
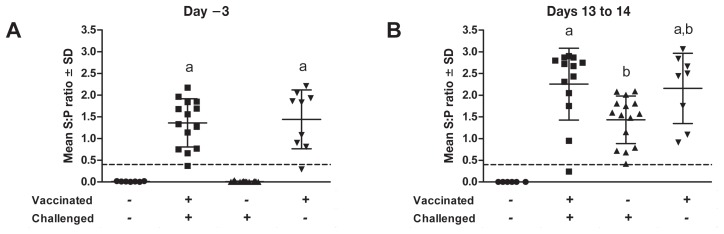
At day 1 after vaccination all the animals were serologically negative for PRRSV (data not shown). At 20 d after vaccination 2 animals, 1 in each of the vaccinated groups, were negative for specific PRRSV antibodies; among the remaining animals the antibody response against PRRSV was similar in the 2 vaccinated groups and significantly greater (P < 0.01) than the response in the nonvaccinated groups (Figure 2A), which indicated that the control animals were naive in regard to PRRSV infection and that a PRRSV-specific immune response was initiated after vaccination. All the nonvaccinated animals challenged with the virulent field strain demonstrated a PRRSV-specific antibody response by day 13 after challenge (Figure 2B), but this response was significantly less than that of the vaccinated-challenged animals on the same day.
Figure 2.
Antibody response to PRRSV in 4 groups of piglets. Blood samples were collected 3 d before challenge (A) and 13 to 14 d after challenge (B). Serum was tested for the presence of specific PRRSV antibodies with HerdChek PRRS X3 diagnostic enzyme-linked immunosorbent assay kits (IDEXX Laboratories, Westbrook, Maine, USA). Data are expressed as the sample-to-positive (S:P) ratio; a ratio of 0.4 or greater is considered positive. Different superscripts indicate a significant difference (P < 0.05) between the groups. SD — standard deviation.
Growth and clinical signs
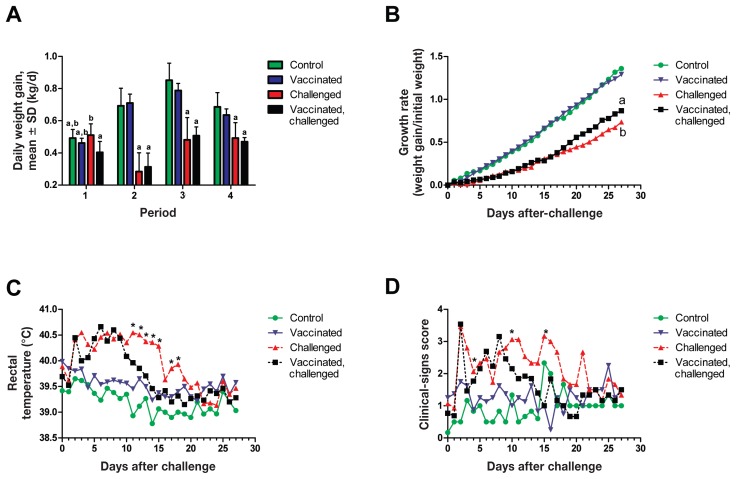
On day 20 after vaccination the ADG of the vaccinated-challenged group was significantly different (P < 0.05) from the ADG of the nonvaccinated-challenged group (Figure 3A). No differences were identified between these 2 groups for any of the 3 other periods, which suggests that the ADG of the vaccinated-challenged animals improved over time. The results for growth rate (Figure 3B) supported that suggestion, as the growth of the vaccinated-challenged animals was significantly greater (P < 0.05) than that of the nonvaccinated-challenged animals from day 24 after challenge until the end of the experiment. The ADGs of both challenged groups, vaccinated and nonvaccinated, were significantly lower (P < 0.05) than those of the 2 nonchallenged groups. Vaccination alone did not significantly affect the growth rate, since the ADG of the vaccinated-nonchallenged group was not significantly different from that of the nonvaccinated-nonchallenged group.
Figure 3.
Growth rates (A, B) and time course of rectal body temperature (C) and clinical signs (D) during infection. “Vaccinated” — vaccinated but not challenged. “Challenged” — not vaccinated but challenged. Average daily weight gain was calculated for each group by dividing the total weight gain in a period of time by the number of days in the period: i) from vaccination (or control inoculation) to challenge; ii) during the first 13 d after challenge (day 0 to day 13); iii) during the first 27 d after challenge; and iv) during the entire period of the experiment. Growth rate was obtained by dividing the weight gain by the initial weight before challenge for each day after challenge. Clinical signs were scored daily according to the system presented in Table I. Different superscripts indicate a significant difference (P < 0.05) between the groups, and an asterisk indicates a significant difference (P < 0.05) between the vaccinated-challenged and the nonvaccinated-challenged groups.
Earlier in the postchallenge period both challenged groups had higher body temperatures than the nonchallenged animals, but from 11 to 18 d after challenge the difference was no longer significant for the vaccinated-challenged animals, which suggests a protective effect of the vaccine. Later on the body temperature of the nonvaccinated-challenged animals was similar to that of the other groups.
The scores for clinical signs after challenge tended to be higher for the nonvaccinated animals compared with the vaccinated animals between 11 to 22 d after challenge and were significantly higher (P < 0.05) on days 3, 11, and 15 (Figure 3D).
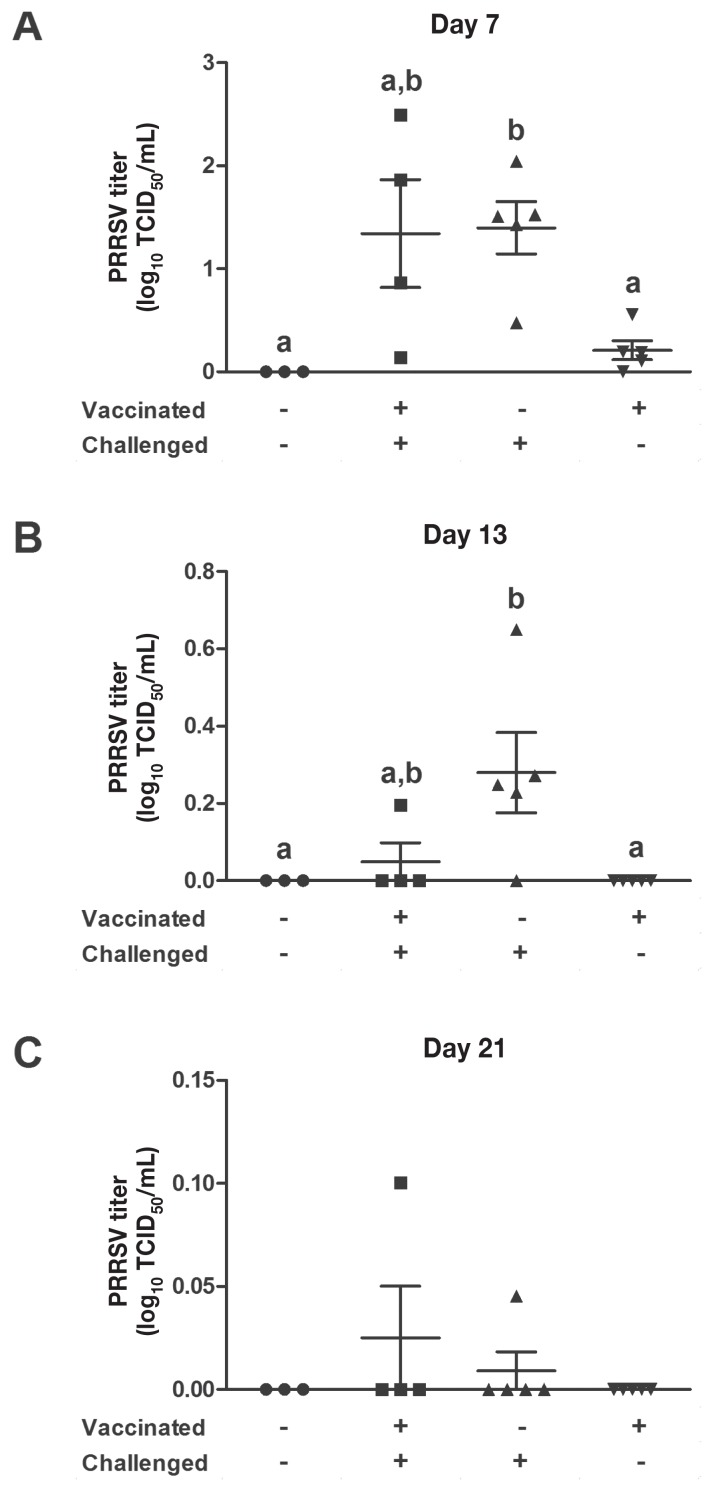
Virologic parameters
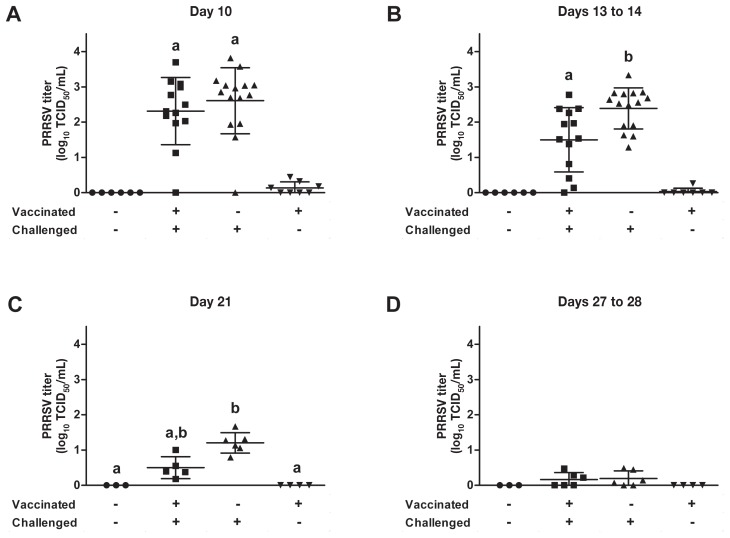
Several PRRSV RT-qPCR diagnostic assays were conducted to establish the impact of vaccination on the level and duration of viremia (Figure 4) and on the persistence of PRRSV in tissues such as the lungs and tracheobronchial lymph nodes (Figure 5). The duration of viremia due to the vaccine strain was established from data for animals that were vaccinated but not challenged. At 36 d after vaccination, only 1 of 8 animals was still viremic, albeit having a very low PRRSV titer (Figure 4B). Thereafter the vaccine strain could not be detected in the vaccinated animals (Figures 4C and 4D). At day 13 after challenge the PRRSV titer was significantly lower (P < 0.001) in the vaccinated animals than in the nonvaccinated animals (Figure 4B). At day 21 after challenge the PRRSV titer was significantly higher (P < 0.05) in the nonvaccinated-challenged animals than in both nonchallenged groups (Figure 4C); however, no significant difference was observed between the 2 challenged groups (Figure 4C). At 27 d after challenge no significant difference was observed between the 4 groups (Figure 4D); nonetheless, several animals in both challenged groups (3 of 6 vaccinated animals and 4 of 6 nonvaccinated animals) were still viremic but with very low titers.
Figure 4.
Level of viremia according to the results of real-time quantitative polymerase chain reaction (RT-qPCR) assay of PRRSV RNA in blood collected on various days after challenge. Different superscripts indicate a significant difference (P < 0.05) between the groups. TCID50 — median tissue culture infective dose.
Figure 5.
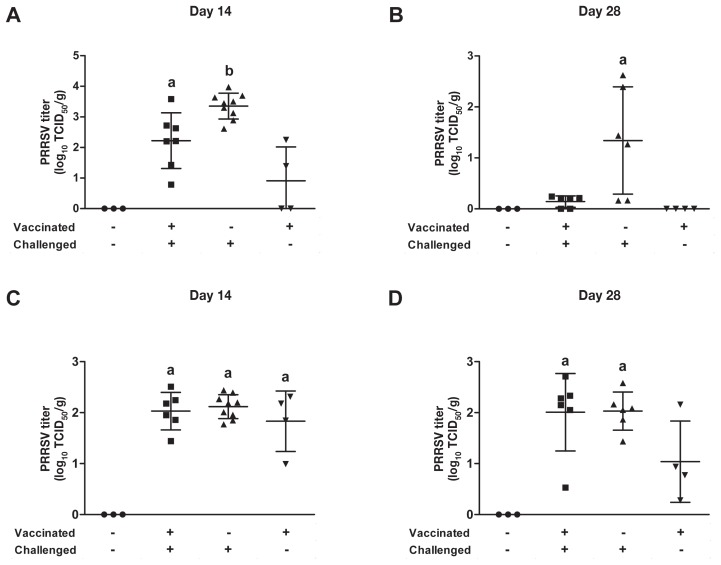
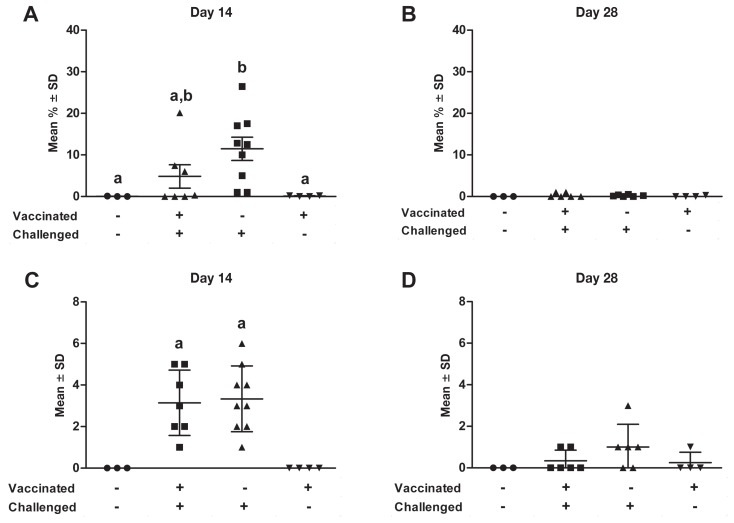
Viral load in the lungs (A, B) and tracheobronchial lymph nodes (C, D) on days 14 and 28 after challenge. Homogenized samples were tested for the presence of PRRSV RNA by RT-qPCR assay. When 2 sets of data are labeled with superscripts of different letters or when only one set is labeled with a superscript, it indicates that these 2 sets of data are statistically different (P < 0.05)
Two vaccinated-nonchallenged animals were positive for PRRSV in lung tissue collected at necropsy 14 d after challenge (Figure 5A), but no animals were positive at 28 d (Figure 5B), which indicates that the vaccine strain did not persist in the lungs more than 50 d after vaccination. However, the vaccine strain was persisting in the lymph nodes at 50 d after vaccination (Figure 5D). Interestingly, the lung viral load was significantly higher (P < 0.05) in nonvaccinated than in vaccinated animals at 14 and 28 d after challenge (Figures 5A and 5B), which suggests that vaccination has an impact on the lung viral load. The viral load of the tracheobronchial lymph nodes was similar in the 2 challenged groups at both times analyzed (Figures 5C and 5D).
Virus shedding
Nasal swabs were collected 7, 13, 21, and 27 d after challenge. Viruses were detected at very low titers on day 7 in 4 of 5 of the vaccinated-nonchallenged animals (Figure 6A). At that time the viral load in nasal swabs was significantly higher (P < 0.05) in the nonvaccinated-challenged animals than in the nonchallenged animals but was not significantly different from the load in the vaccinated-challenged animals. At 13, 21, and 27 d after challenge all the nasal swabs of the vaccinated-nonchallenged animals were PRRSV-negative (Figures 6B and 6C and data not shown, respectively). At 13 d after challenge the viral load in the nasal swabs was lower in the vaccinated-challenged animals than in the nonvaccinated-challenged animals, but the differences were not statistically significant (P = 0.11) (Figure 6B). Still, the nasal viral load was higher in the nonvaccinated-challenged animals than in the nonchallenged animals (P < 0.05). At day 27 after challenge all the tested nasal swabs were negative (data not shown).
Figure 6.
Nasal virus shedding. Nasal swabs were collected on days 7 (A), 13 (B), and 21 (C) after challenge and tested for the presence of PRRSV RNA by RT-qPCR assay. When 2 sets of data are labeled with superscripts of different letters or when only one set is labeled with a superscript, it indicates that these 2 sets of data are statistically different (P < 0.05).
Lung lesions
There were no significant differences between the 2 challenged groups in the mean percentage of lung tissue with macroscopic lesions at necropsy (Figures 7A and 7B). The lesions tended to be more extensive in the nonvaccinated group at day 14 after challenge (Figure 7A) (P = 0.071 after arcsine and square root transformation of the percentage data). In 50% of the vaccinated-challenged animals either there were no macroscopic lung lesions or less than 1% of the lung was affected; furthermore, this group was not significantly different from the nonvaccinated-nonchallenged group in terms of macroscopic lesions (Figure 7A). At 14 d after challenge (Figure 7A) the proportion of nonvaccinated-challenged animals that had macroscopic lung lesions was high, 67% of this group having a score of 10% or more; in addition, this group had significantly higher scores (P < 0.05) than the 2 nonchallenged groups. At 28 d after challenge (Figure 7B) very few macroscopic lung lesions were observed at necropsy in the challenged animals, which indicates that the lungs of even the nonvaccinated animals were healing; thus, no significant difference between the experimental groups was observed.
Figure 7.
Percentage of lung with macroscopic lesions (A,B) on days 14 and 28 after challenge and score for microscopic lung lesions (C,D) on the same days. When 2 sets of data are labeled with superscripts of different letters or when only one set is labeled with a superscript, it indicates that these 2 sets of data are statistically different (P < 0.05).
Overall the histopathological findings were in accord with the macroscopic findings in the lung tissue. Histopathologically the lesions were more extensive at 14 d after challenge compared with 28 d. The PRRSV-specific lesions were characterized by septal thickening and alveolar necrotic debris, macrophages, and other mononuclear cells. No significant differences were found between the 2 challenged groups at 14 and 28 d (P = 0.91 and 0.25, respectively) (Figures 7C and 7D). However, at 14 d the lesion score was significantly higher for all the challenged animals than for the non-challenged animals (P = 0.02 and 0.01).
Discussion
The efficacy of PRRSV MLV vaccines depends greatly on the degree of genetic similarity between the vaccine and challenge strains, but the degree of ORF5 homology between the strains is not always a good predictor of the immune response (21,22). However, it is well-accepted that immunity against genetically related strains is almost completely sterilizing, whereas the immunity against genetically divergent strains is more variable (23). In this study, PRRSV ORF5 genomic analysis revealed that the amino acid homology between the vaccine and challenge strains was 86%. This level of identity clearly illustrates that the 2 strains were heterologous, and this divergence may affect the vaccine’s cross-protective efficacy. In a previous study Fostera PRRS showed some efficacy in reducing the level of viremia and the severity of PRRSV-induced lesions after challenge with a Korean heterologous strain that shared, according to our evaluation, 88.4% of the ORF5 amino acid sequence with the vaccine strain (17). The level of heterogeneity in the present study is similar; however, results from the previous study may not be predictive of the effectiveness of the vaccine since amino acid homology between the Korean and the Canadian strains is only 91.5% (17). To evaluate the efficacy of the Fostera PRRS vaccine in a Canadian context, piglets were vaccinated and subsequently infected with a PRRSV heterologous strain that is widely circulating in the swine herds of eastern Canada.
Porcine reproductive and respiratory syndrome virus can cause many clinical manifestations, including anorexia, fever, lethargy, and severe pneumonia, often complicated by concurrent bacterial infection, as well as a reduction in weight gain (24,25). In this study, vaccination with an attenuated PRRSV strain resulted in a decrease in ADG over a 3-week period after vaccination of approximately 14%. This loss is of the same magnitude as has been shown for other MLV vaccines (22,26). The reduction in weight gain decreased from 14% to 8% at day 50, suggesting compensation. The reduction in weight gain caused by the field strain used for experimental infection was much more severe, about 44%. Vaccination did not have a significantly positive impact on weight gain when one considers the entire period of the experiment. This could be explained by the short interval between vaccination and challenge. Previous reports argued that maximum heterologous protection could be reached at least 5 wk after vaccination (22,27). However, in the present study, Fostera PRRS vaccination increased the growth rate during the post-challenge period by 20%. In terms of growth rate the vaccinated pigs clearly had an advantage starting at day 24 after challenge, which suggests a delayed-type immune response. The same conclusion can be drawn in terms of body temperature and clinical signs. Indeed, the body temperature declined more rapidly in vaccinated animals starting at day 11 after challenge. The high body temperature lasted about 1 wk longer in the nonvaccinated animals.
In growing pigs infected with a PRRSV genotype II strain, the intensity of clinical signs usually correlates well with viremia (28,29). Thus, a protective effect can be inferred from the level of viremia (30). In our study the level of viremia did not differ significantly between the vaccinated and nonvaccinated animals except at day 13 after challenge, when the level was significantly lower in the vaccinated animals. Similar results have been obtained previously for heterologous protection against virulent strains of genotype II (31). This suggests that virus elimination starts earlier in vaccinated animals. At day 21 after challenge the infection had almost completely resolved in most of the vaccinated animals in our study (4 of 5) compared with few of the nonvaccinated animals (1 of 6). In addition, the lung viral load was significantly lower in the vaccinated animals at day 14 after challenge. These results support the previous conclusion that MLV vaccines could be used to reduce virus shedding in the environment (32). In the present study, no PRRSV vaccine strains were detected in serum, lung tissue, or nasal swabs starting at 44, 50 to 51, and 36 d after vaccination, respectively, which suggests that the risk of vaccine virus shedding will be low from day 50 to 51 after vaccination. It is noteworthy that the tracheobronchial lymph nodes were still PRRSV-positive on the last experimental day in all groups except the nonvaccinated-nonchallenged animals. Other reports have also indicated that PRRSV persists longer in lymph nodes than in blood and lungs (33,34). The Fostera PRRS vaccine strain is not an exception since at the end of the experiment (50 to 51 d after vaccination) PRRSV could still be detected in lymph nodes but not serum, lung tissue, or nasal swabs from all the vaccinated-nonchallenged animals in our study. However, the viral load of the vaccine strain within the lymph nodes was significantly lower in the vaccinated-nonchallenged animals than in the challenged animals, which indicates lower virulence of the vaccine strain compared with PRRSV FMV12-1425619.
Porcine reproductive and respiratory syndrome virus is responsible for specific lung lesions that vary from no apparent lesions to severe tan consolidation that is frequently aggravated by lesions resulting from concurrent bacterial infection (25). At day 14 after challenge in this study the lung lesions were less extensive in the vaccinated animals, suggesting again a partial protective effect.
Fostera PRRS did not confer complete protection against disease induced by the heterologous PRRSV Canadian strain used in this study, but overall the vaccine showed some beneficial effects, reducing the severity of clinical signs, body temperature, the level of viremia, and the pulmonary viral load. A significant difference between nonvaccinated and vaccinated animals was detected for some parameters 11 to 13 d after challenge, which suggests that cell-mediated immune response or other delayed responses could play a more important role than the pre-existing PRRSV antibodies in vaccinated animals in the context of heterologous vaccine protection. However, neutralizing antibodies appear only 28 d after the onset of infection (35). Since the challenge was done 21 d after vaccination, we cannot exclude the possibility that neutralizing antibodies played a role in the positive impact of the vaccine. A previous report attributed heterologous cross-protection to cell-mediated immunity (36).
Acknowledgments
The authors thank Guy Beauchamp for his advice on statistical analysis. This project was financially supported by Zoetis Canada. Drs. Savard and Provost received postdoctoral fellowships from the Canadian Swine Health Board, Dr. Savard received a postdoctoral fellowship from Fonds de recherche du Québec — Nature et technologies, and Dr. Alvarez received a graduate scholarship from Fédération des producteurs de porcs du Québec. Dr. Gagnon was financially supported by the Natural Sciences and Engineering Research Council of Canada. The animal facility infrastructure used for this project was financially supported by a Canada Foundation for Innovation (CFI) infrastructure grant.
References
- 1.Holtkamp DJ, Kliebenstein JB, Neumann EJ, et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Prod. 2013;21:72–84. [Google Scholar]
- 2.Chand RJ, Trible BR, Rowland RR. Pathogenesis of porcine reproductive and respiratory syndrome virus. Curr Opin Virol. 2012;2:256–263. doi: 10.1016/j.coviro.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Dorr PM, Gebreyes WA, Almond GW. Porcine reproductive and respiratory syndrome virus: Age and management system disease modeling for pathogenic co-infection. J Swine Health Prod. 2007;15:258–263. [Google Scholar]
- 4.Meulenberg JJ, Hulst MM, de Meijer EJ, et al. Lelystad virus belongs to a new virus family, comprising lactate dehydrogenase- elevating virus, equine arteritis virus, and simian hemorrhagic-fever virus. Arch Virol Suppl. 1994;9:441–448. doi: 10.1007/978-3-7091-9326-6_43. [DOI] [PubMed] [Google Scholar]
- 5.Music N, Gagnon CA. The role of porcine reproductive and respiratory syndrome (PRRS) virus structural and non- structural proteins in virus pathogenesis. Anim Health Res Rev. 2010;11:135–163. doi: 10.1017/S1466252310000034. [DOI] [PubMed] [Google Scholar]
- 6.Kimman TG, Cornelissen LA, Moormann RJ, Rebel JMJ, Stochofe-Zurwieden N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine. 2009;27:3704–3718. doi: 10.1016/j.vaccine.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Shi M, Lam TT, Hon CC, et al. Phylogeny-based evolutionary, demographical, and geographical dissection of North American type 2 porcine reproductive and respiratory syndrome viruses. J Virol. 2010;84:8700–8711. doi: 10.1128/JVI.02551-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh Brar M, Shi M, Ge L, Carman S, Murtaugh MP, Leung FC. Porcine reproductive and respiratory syndrome virus in Ontario, Canada 1999 to 2010: Genetic diversity and restriction fragment length polymorphisms. J Gen Virol. 2011;92(Pt 6):1391–1397. doi: 10.1099/vir.0.030155-0. [DOI] [PubMed] [Google Scholar]
- 9.Delisle B, Gagnon CA, Lambert ME, D’Allaire S. Porcine reproductive and respiratory syndrome virus diversity of Eastern Canada swine herds in a large sequence dataset reveals two hypervariable regions under positive selection. Infect Genet Evol. 2012;12:1111–1119. doi: 10.1016/j.meegid.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Shi M, Lemey P, Singh Brar M, et al. The spread of type 2 porcine reproductive and respiratory syndrome virus (PRRSV) in North America: A phylogeographic approach. Virology. 2013;447:146–154. doi: 10.1016/j.virol.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Dea S, Gagnon CA, Mardassi H, Milane G. Antigenic variability among North American and European strains of porcine reproductive and respiratory syndrome virus as defined by monoclonal antibodies to the matrix protein. J Clin Microbiol. 1996;34:1488–1493. doi: 10.1128/jcm.34.6.1488-1493.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wensvoort G, de Kluyver EP, Pol JM, et al. Lelystad virus, the cause of porcine epidemic abortion and respiratory syndrome: A review of mystery swine disease research at Lelystad. Vet Microbiol. 1992;33:185–193. doi: 10.1016/0378-1135(92)90046-v. [DOI] [PubMed] [Google Scholar]
- 13.Andreyev VG, Wesley RD, Mengeling WL, Vorwald AC, Lager KM. Genetic variation and phylogenetic relationships of 22 porcine reproductive and respiratory syndrome virus (PRRSV) field strains based on sequence analysis of open reading frame 5. Arch Virol. 1997;142:993–1001. doi: 10.1007/s007050050134. [DOI] [PubMed] [Google Scholar]
- 14.Labarque G, Reeth KV, Nauwynck H, Drexler C, Van Gucht S, Pensaert M. Impact of genetic diversity of European-type porcine reproductive and respiratory syndrome virus strains on vaccine efficacy. Vaccine. 2004;22:4183–4190. doi: 10.1016/j.vaccine.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Pesch S, Meyer C, Ohlinger VF. New insights into the genetic diversity of European porcine reproductive and respiratory syndrome virus (PRRSV) Vet Microbiol. 2005;107:31–48. doi: 10.1016/j.vetmic.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 16.Murtaugh MP, Genzow M. Immunological solutions for treatment and prevention of porcine reproductive and respiratory syndrome (PRRS) Vaccine. 2011;29:8192–8204. doi: 10.1016/j.vaccine.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Park C, Seo HW, Han K, Kang I, Chae C. Evaluation of the efficacy of a new modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (Fostera PRRS) against heterologous PRRSV challenge. Vet Microbiol. 2014;172:432–442. doi: 10.1016/j.vetmic.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Olfert ED, Cross BM, McWilliam AA, editors. Guide to the Care and Use of Experimental Animals. 2nd ed. Vol. 1. Ottawa, Ontario: Canadian Council on Animal Care; 1993. [Last accessed October 29, 2015]. Available from: www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf. [Google Scholar]
- 19.Gagnon CA, del Castillo JR, Music N, Fontaine G, Harel J, Tremblay D. Development and use of a multiplex real-time quantitative polymerase chain reaction assay for detection and differentiation of porcine circovirus-2 genotypes 2a and 2b in an epidemiological survey. J Vet Diagn Invest. 2008;20:545–558. doi: 10.1177/104063870802000503. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen V, Jorsal SE, Mousing J. Diseases of the respiratory system. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, editors. Diseases of Swine. Ames, Iowa: Blackwell Publishing; 2006. pp. 149–177. [Google Scholar]
- 21.Prieto C, Alvarez E, Martinez-Lobo FJ, Simarro I, Castro JM. Similarity of European porcine reproductive and respiratory syndrome virus strains to vaccine strain is not necessarily predictive of the degree of protective immunity conferred. Vet J. 2008;175:356–363. doi: 10.1016/j.tvjl.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Opriessnig T, Pallares FJ, Nilubol D, et al. Genomic homology of ORF 5 gene sequence between modified live vaccine virus and porcine reproductive and respiratory syndrome virus challenge isolates is not predictive of vaccine efficacy. J Swine Health Prod. 2005;13:246–253. [Google Scholar]
- 23.Díaz I, Gimeno M, Darwich L, et al. Characterization of homologous and heterologous adaptive immune responses in porcine reproductive and respiratory syndrome virus infection. Vet Res. 2012;43:30. doi: 10.1186/1297-9716-43-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Done SH, Paton DJ, White MEC. Porcine reproductive and respiratory syndrome (PRRS): A review, with emphasis on pathological, virological and diagnostic aspects. Br Vet J. 1996;152:153–174. doi: 10.1016/S0007-1935(96)80071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossow KD. Porcine reproductive and respiratory syndrome. Vet Pathol. 1998;35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- 26.Mavromatis I, Kritas SK, Alexopoulos C, Tsinas A, Kyriakis SC. Field evaluation of a live vaccine against porcine reproductive and respiratory syndrome in fattening pigs. Zentralbl Veterinarmed B. 1999;46:603–612. doi: 10.1046/j.1439-0450.1999.00282.x. [DOI] [PubMed] [Google Scholar]
- 27.Mengeling WL, Lager KM, Vorwald AC, Clouser DF. Comparative safety and efficacy of attenuated single-strain and multi-strain vaccines for porcine reproductive and respiratory syndrome. Vet Microbiol. 2003;93:25–38. doi: 10.1016/s0378-1135(02)00426-1. [DOI] [PubMed] [Google Scholar]
- 28.Johnson W, Roof M, Vaughn E, Christopher-Hennings J, Johnson CR, Murtaugh MP. Pathogenic and humoral immune responses to porcine reproductive and respiratory syndrome virus (PRRSV) are related to viral load in acute infection. Vet Immunol Immunopathol. 2004;102:233–247. doi: 10.1016/j.vetimm.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Klinge KL, Vaughn EM, Roof MB, Bautista EM, Murtaugh MP. Age-dependent resistance to porcine reproductive and respiratory syndrome virus replication in swine. Virol J. 2009;6:177. doi: 10.1186/1743-422X-6-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opriessnig T, Baker RB, Halbur PG. Use of an experimental model to test the efficacy of planned exposure to live porcine reproductive and respiratory syndrome virus. Clin Vaccine Immunol. 2007;14:1572–1577. doi: 10.1128/CVI.00332-07. Epub 2007 Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roca M, Gimeno M, Bruguera S, et al. Effects of challenge with a virulent genotype II strain of porcine reproductive and respiratory syndrome virus on piglets vaccinated with an attenuated genotype I strain vaccine. Vet J. 2012;193:92–96. doi: 10.1016/j.tvjl.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Linhares DC, Cano JP, Wetzell T, Nerem J, Torremorell M, Dee SA. Effect of modified-live porcine reproductive and respiratory syndrome virus (PRRSv) vaccine on the shedding of wild-type virus from an infected population of growing pigs. Vaccine. 2011;30:407–413. doi: 10.1016/j.vaccine.2011.10.075. [DOI] [PubMed] [Google Scholar]
- 33.Rowland RR, Lawson S, Rossow K, Benfield DA. Lymphoid tissue tropism of porcine reproductive and respiratory syndrome virus replication during persistent infection of pigs originally exposed to virus in utero. Vet Microbiol. 2003;96:219–235. doi: 10.1016/j.vetmic.2003.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wills RW, Doster AR, Galeota JA, Sur JH, Osorio FA. Duration of infection and proportion of pigs persistently infected with porcine reproductive and respiratory syndrome virus. J Clin Microbiol. 2003;41:58–62. doi: 10.1128/JCM.41.1.58-62.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez OJ, Osorio FA. Role of neutralizing antibodies in PRRSV protective immunity. Vet Immunol Immunopathol. 2004;102:155–163. doi: 10.1016/j.vetimm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Martelli P, Gozio S, Ferrari L, et al. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine. 2009;27:3788–3799. doi: 10.1016/j.vaccine.2009.03.028. [DOI] [PubMed] [Google Scholar]