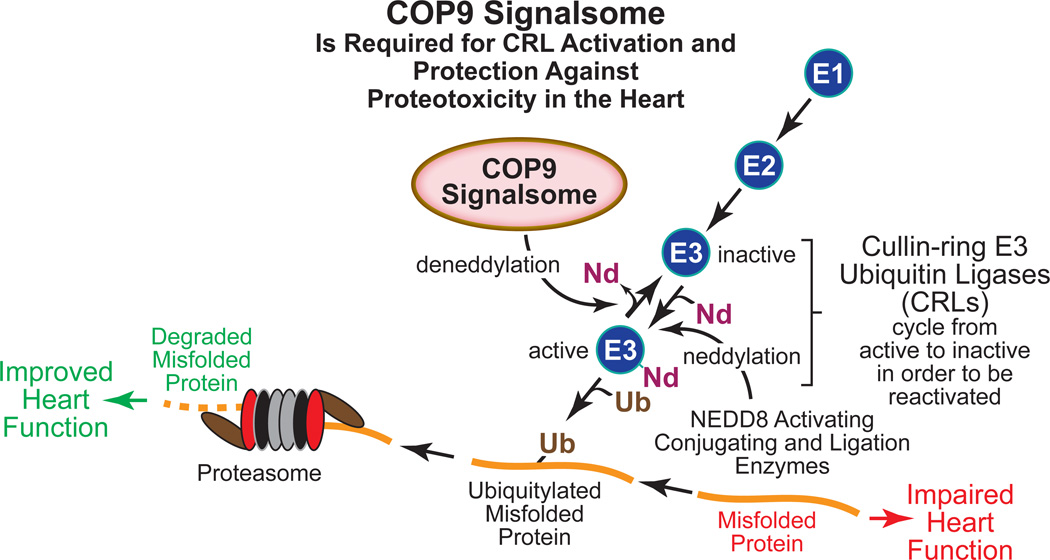
Figure. The COP9 Signalsome Activates Cullin-ring E3 Ubiquitin Ligases and Protects the Heart from Proteotoxicity.
Various cardiac pathologies are associated with accumulation of misfolded protein in cardiac myocytes, which due to proteotoxicity, impairs heart function. The cullin-ring E3 ubiquitin ligases (CRLs) constitute the largest group of the E3 ubiquitin ligases in all cell types; thus, CRLs are responsible for recognizing a large proportion of misfolded toxic proteins and targeting them for proteasome-mediated degradation by ubiquitylation. This removal of misfolded toxic proteins can improve heart function. CRL activation requires neddylation, i.e. covalent addition of the ubiquitin-like 81 amino acid protein, NEDD8 (Nd) by NEDD8-specific activating, conjugation and ligation enzymes, much like those used by the ubiquitylation machinery. But in order to regain the ability to bind a new substrate for ubiquitylation, CRLs must undergo COP9 signalsome (CSN)-mediated deneddylation. The study by Su et al in this issue of Circulation Research has shown that in a mouse model of proteinopathy in the heart, the CSN supports increased ubiquitylation of misfolded proteins and improves cardiac function.