Abstract
Radiofrequency ablation (RFA) is widely accepted as a first-line interventional oncology approach for hepatocellular carcinoma (HCC) and has the advantages of high treatment efficacy and low complication risk. Local control rates equivalent to hepatic resection can be reached by RFA alone when treating small HCCs (<2 cm) in favorable locations. However, local tumor progression and recurrence rates with RFA monotherapy increase sharply when treating larger lesions (>3 cm). To address this clinical problem, recent efforts have focused on multimodel management of HCC by combining RFA with different techniques, including percutaneous ethanol injection, transarterial chemo-embolization, targeted molecular therapy, nanoparticle-mediated therapy, and immunotherapy. The combination strategy indeed leads to better outcomes in comparison to RFA alone. In this article, we review the current status of RFA-combined multimodal therapies in the management of HCC.
Keywords: Hepatocellular carcinoma, Radiofrequency ablation, Interventional oncology, Multimodel treatment
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death, with increasing incidence worldwide. Population-based studies reveal that the incidence rate is close to the death rate, indicative of its high fatality rate [1,2]. Treatment of this malignancy should be carefully selected based on disease stages. Currently, The Barcelona Clinic Liver Cancer (BCLC) decision-making strategy is one of the most widely accepted staging systems. In the current clinical practice, hepatic resection (HC), liver transplantation (LT), radiofrequency ablation (RFA), transhepatic arterial chemoembolization (TACE) and sorafenib are the main therapeutic modalities (Table 1).
Table 1.
| Modalities | Status | Indication | 5-year OS (%) |
|---|---|---|---|
| HR | Best treatment for non-cirrhosis patients. | BCLC stage 0/A | 37–53 |
| LT | Best treatment for cirrhotic patients. | BCLC stage 0/A | 38.2–46.5 |
| RFA | Comparable to HR in small HCCs. | BCLC stage 0/A | 33–60.2 |
| TACE | Standard of care for patients with large or multi-nodular HCC. | BCLC stage B | 26–34 |
| Sorafenib | The only approved systemic therapy. | BCLC stage C | 2–3 month OS prolongation |
HR = hepatic resection; LT = liver transplantation; RFA = radiofrequency ablation; TACE = transhepatic arterial chemoembolization; OS = overall survival; BCLC = Barcelona Clinic Liver Cancer.
HC and LT offer the best prognoses in patients with early stage Q3 HCC [8]. Unfortunately, due to the asymptomatic nature of this disease, more than 80% of patients have already lost the opportunity for surgical removal of tumors by the time of diagnosis [9]. Furthermore, patients undergoing surgical treatment usually have high cancer recurrence rates of 79.4% and 92.5% at 3 and 5 years after surgery, respectively [10]. Additionally, a shortage of LT donors remains a medical difficulty for LT candidates [8,11].
Image-guided loco-regional ablation therapies, such as RFA, percutaneous ethanol injection (PEI), microwave ablation, cryoablation, high-intensity focused ultrasound ablation, and irreversible electroporation currently play key roles in the management of unresectable lesions. Among these ablation techniques, RFA is currently recognized as the main ablative tool for HCC tumors < 5 cm in size. RFA also functions as a bridge to transplant treatment [12,13]. According to the updated clarifications outlined by the BCLC system, patients diagnosed at a very early stage (BCLC 0) with a marginal risk of recurrence should be considered for surgery only if a transplant is available; if not, RFA should be the first-line option, while surgical resection is justified only for those patients who have experienced failure of, or contraindication to, RFA [3].
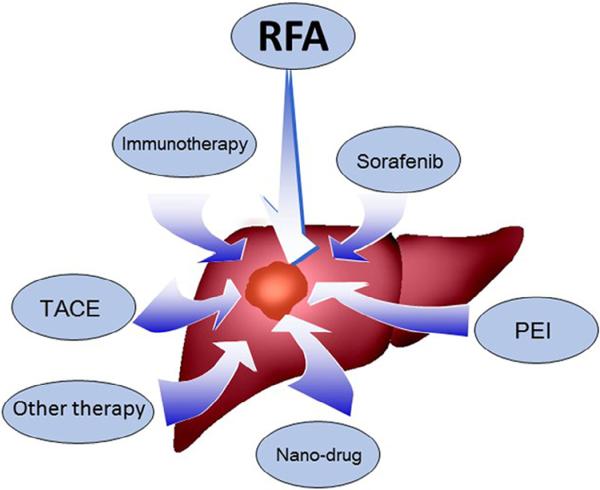
The rates of local tumor recurrence and progression following RFA treatment increase sharply when treating larger lesions; this remains a major problem with the RFA technique [14]. In the current clinical practice, RFA is not recommended for tumors larger than 5 cm, owing to (a) the limited treatment area affected by an RF electrode in a large tumor; (b) the presence of microscopic vascular invasion or satellites around more advanced large malignant tumors; (c) the fact that irregularly shaped tumors are not completely covered by the radiofrequency (RF) energy; and (d) insufficient ablative coagulation necrosis caused by the heat-sink effect, which means heat deposition is limited by the cooling effect due to blood flows of the larger vessels [15–17]. Usually, a minimum of a 360°, 0.5–1 cm-sized, circumferential ablation of peri-tumoral tissue is required in clinical applications. It is believed that complete ablation of HCCs up to 4–5 cm in size can be achieved by using more advanced RFA systems or overlapping ablation strategies [15,18]. However, more aggressive treatments mean an increased risk of injuries associated with unwanted thermal damage to important normal structures, such as the bile duct, gallbladder, diaphragm, and intestinal tracts [19,20]. Thus, in order to take full advantage of RFA in HCC management, recent efforts have focused on the combination of RFA with other anti-cancer approaches, including PEI, transarterial chemoembolization (TACE), nanoparticle-mediated therapy, molecular targeted therapy, and immunotherapy (Fig. 1). The concept of multimodal therapeutic strategies has become a clinical reality in the effective treatment of HCC [21,22]. In this article, we review the current status of RFA-combination multimodal therapies in HCC management.
Fig. 1.
Major multimodal strategies for RFA-combined therapies of HCCs. Combination of RFA with TACE and PEI has been established as a clinical strategy for treating HCC, while Sorafenib and Nano-drug (ThermoDox®) have entered Phase III clinical trial stage in combination with RFA. Combination of RFA with immunotherapy is still in preclinical stage.
Mechanism of RFA
Since first reported in 1993 [23], RFA has been widely applied in the management of HCC. RFA delivers a high frequency alternating current through a needle-electrode into the tumor. This results in ionic agitation caused by the alternating electric currents within the tumor, which subsequently causes friction heat; this friction heat results in the ablation of target tumor tissues [24]. HCC in cirrhotic liver is an ideal indication for RFA because the heat energy can be effectively insulated by cirrhotic tissue surrounding the tumor, the so-called “oven effect” [25].
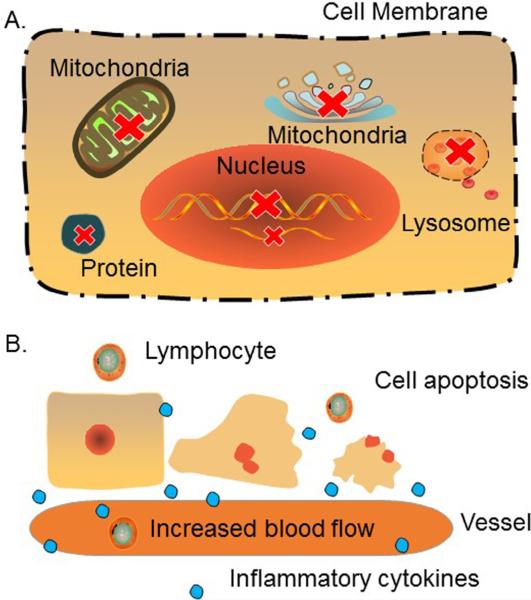
A complex of biochemical reactions occurs during and after the RFA procedure. Pathologically, a heat-ablated tumor lesion can be divided into three zones: (a) a central necrotic zone directly around the needle-electrode, where protein denaturation occurs rapidly; (b) a peripheral zone that undergoes sub-lethal hyperthermia; and (c) outermost tissues that are unaffected by RFA [26,27]. It has been demonstrated that when tissue is heated to approximately 50 °C for 4–6 minutes, irreversible damage can be done to the cells. Therefore, 50 °C is generally accepted as the low cutoff temperature for RFA, the so-called “critical temperature” [28,29]. The mechanisms of direct thermal damage in the central necrotic zone are rather complex. The basis for this complexity can be found in several biological pathways: the impairment of cell membrane and cytoskeleton function; dysfunction of the mitochondrial and Golgi apparatus; inhibition of DNA replication and RNA synthesis; and the release of lysosomal enzymes [27]. Indirect or delayed tumor injury happens in the peripheral zone with a thermal burden <50 °C, which is often associated with cell apoptosis, vascular damage, and an immune response induced by RFA [27] (Fig. 2). Tumor cells in the peripheral zone become more vulnerable to chemotherapy and radiotherapy [26,27,30,31].
Fig. 2.
Biological reactions with RFA procedure. (A) Necrosis zone: When tissue is heated >50 °C for several minutes, several biologic reactions occur, including cell membrane collapse, protein denaturation, DNA replication and RNA synthesis inhibition, as well as dysfunctions of mitochondrial, lysosome and Golgi. (B) Sub-lethal zone: With a thermal burden <50 °C, tumor damage is associated with cell apoptosis. Vascular damage along with increase of blood in this area contributes to the accumulation of inflammatory cytokine and lymphocyte, which thereby induce immune responses.
RFA-combined multimodal therapies
RFA plus PEI
PEI is considered a relatively safe, simple, and low-cost chemical ablation technology. By diffusing 95% ethyl alcohol into the tumor parenchyma, PEI can cause dehydration in tumor cells, and together with microvascular thrombosis, result in tumor ischemia and destruction [32]. PEI has been reported to be less effective than RFA and has essentially been replaced by RFA in recent years [9]. However, in special circumstances PEI has an important therapeutic role. Up to 25% of small liver tumors are located in adjacent normal structures, such as the portal vein, bile ducts, gallbladder or the subcapsular areas in contact with the diaphragm and intestinal tracts [33]; the efficacy of RFA decreases since the operators have to carefully consider the balance between sufficient ablation of lesions and avoiding collateral thermal injury to these vital structures. Under these circumstances, PEI can be considered a better strategy [34].
To achieve a more complete ablation of tumor tissue, the use of PEI is recommended to strengthen the effect of RFA. Several studies have shown that the RFA-PEI combination strategy can create a larger ablative zone than RFA alone [35–39]. The efficacy of this treatment can be explained as follows: (a) PEI can reduce the heat-sink effect by destroying tumor vessels and enhancing thermal conduction in the tumor tissues; (b) ethanol heated by RFA results in better therapeutic efficiency than cool ethanol; and (c) diffusion of ethanol into areas where RF energy is inefficient improves the efficiency [40].
The therapeutic efficacy of the PEI–RFA combination has been evaluated by several clinical studies (Table 2). These studies have found that PEI–RFA treatment is superior to RFA alone in the treatment of HCCs > 3 cm in size or HCC near vital structures. Some authors further demonstrated that combined PEI–RFA therapy can also reduce the RF energy required to induce coagulated necrosis levels [39]. Another study showed that a 5-minute time lag between the two procedures makes the combination therapy more effective and less invasive [46]. The direct injection of a mixture of ethanol and lipiodol allows better visualization of RF-ablated safe margins in RFA–PEI combination therapy [47], and the placement of multiple injection needles can achieve a more homogeneous ethanol distribution, further improving the therapeutic efficacy [41]. Technically, while the sequence protocol of PEI first followed by RFA was described by most of the studies, the performance of RFA prior to PEI has also been described [38].
Table 2.
Survival outcomes for PEI-RFA treatment compared with RFA treatment only.
| Treatment | Tumor number | Tumor diameter (cm) | Overall survival (years) |
||
|---|---|---|---|---|---|
| 1 | 3 | 5 | |||
| PEI-RFA [41–45] | 22 | 2–4 | 92.2% | 81% | 67.7% |
| 33 | ≤5 | 96.7% | NA | NA | |
| 40 | >4 | 92% | 83% | NA | |
| 67 | 3.1–7 | 93.1% | NA | NA | |
| 25 | 3.1–5 | 96% | 77.6% | 55.6% | |
| RFA [7] | 281 | >3 | 94.8% | 71% | 46.5% |
NA = not available; PEI-RFA = percutaneous ethanol injection-radiofrequency ablation; RFA = radiofrequency ablation.
RFA plus TACE
TACE is another common interventional oncological treatment for unresectable HCC [48]. TACE works by blocking blood flow from the arteries that supply the liver tumors, via arterial injection of chemotherapeutics and embolization agents [49,50]. The synergy between RFA and TACE produces the following results: (a) arterial flows are reduced after TACE, which leads to the reduction of the heat-sink effect with RFA [51]; (b) heat deposition in the sub-lethal area during the RFA procedure can enhance the effect of anti-tumor chemotherapeutics used in TACE and thus results in improved ablation of peri-tumoral areas where micro-metastasis could exist [30,52]; (c) TACE can destroy intra-tumoral septa, which improves heat distribution within the tumor [53]; (d) TACE can block tumor-supplying arteries, resulting in tumor volume shrinkage prior to RFA [54]; (e) TACE enhances the conspicuity of HCC nodules on navigation systems and thus facilitates RFA [55]; and (f) TACE is theoretically believed to decrease the risk of neoplastic seeding caused by punctures during the RFA procedure [49,56].
One meta-analysis study demonstrated that an improved 5-year survival rate can be achieved with the combined TACE–RFA treatment in patients with small (<3 cm) HCC masses [57]. Another meta-analysis suggested that RFA–TACE was more effective than RFA monotherapy with higher overall and recurrence-free survival rates in patients with more advanced HCC [58]. An additional study demonstrated that it was safe to treat HCCs < 5 cm in size in cirrhotic patients at a short term interval of 0–2 days with a combination of TACE and RFA treatments [59]. However, a standardized protocol for this combination therapy, including optimization of treatment sequences (TACE–RFA or RFA–TACE), as well as repeat times and time-intervals between the two treatments, is warranted. The appropriate protocol should achieve the synergistic effect of TACE–RFA, with no associated liver function damage.
RFA plus targeted molecular therapy
Targeted molecular therapy is a recent advancement in modern personalized medicine. Most targeted molecular agents work by inhibiting kinases involved in tumor angiogenesis; angiogenesis is highly associated with local tumor progression as well as tumor invasion and metastasis [60]. Hypoxia, an important precipitating factor for angiogenesis, can be induced by incomplete tumor ablation. It has been demonstrated that the level of hypoxia-inducible factor-1α, an important factor in the transcription of multiple angiogenesis-related genes, is increased in residual cancer following RFA [61]. Thus, these anti-angiogenesis-targeted molecular agents may inhibit the growth of the residual tumor when used in combination with RFA. Additionally, similar to the TACE–RFA approach, application of targeted molecular treatments prior to RFA can reduce the blood flow to HCC masses [62], thereby reducing the adverse impact of the heat-sink effect during RFA.
RFA plus sorafenib
Sorafenib, an active multi-kinase inhibitor, is currently the only targeted molecular agent approved by the United States Food and Drug Administration (FDA) for the treatment of patients with advanced HCC; it can prolong overall survival by 2–3 months [63]. The mechanism of sorafenib in the treatment of HCCs is reported to activate several pathways, especially the signaling pathways of Raf/MEK/ERK, vascular endothelial growth factor receptor, platelet-derived growth factor receptor, as well as c-KIT and RET. These pathways are closely related to the pathogenesis of HCC [64–67]. Some scientists compared the coagulation induced with RFA alone and that with sorafenib prior to RFA in a renal cell carcinoma mouse model. Significantly larger volumes of coagulation were seen in the sorafenib–RFA group compared with the control groups [68]. Similar results were found in a rat model [69]. Another study established a two-tumor rat model of HCC to study the effect of combination sorafenib–RFA treatment (i.e. one tumor treated by RFA and another untreated as a control). They found a significant reduction in volume and a more pronounced destruction in the non-RFA treated tumor with a combination of the two therapies [70]. This demonstrates that the combination of sorafenib and RFA can enhance the effect of RFA ablation on target tumors and that it has the potential to increase the killing effect of sorafenib on non-RFA-treated tumor tissues. Recently, some authors retrospectively compared the levels of coagulation induced by RFA alone and sorafenib–RFA in the treatment of HCCs < 3 cm. RFA was performed 7 days after administration of sorafenib. This study showed that the size of the ablated area was significantly larger in the sorafenib–RFA group than in the control group [71]. Other authors compared the clinical prognosis of RFA-sorafenib treatment and RFA treatment alone in 62 patients with hepatitis B virus-related medium-sized HCC (3.1–5.0 cm in diameter). This study revealed a decreased recurrence rate and prolonged survival time with the combination treatment [72]. By analyzing 128 patients with HCC at different stages of BCLC (0-B1), a recent study concluded that improved 1-, 2-, and 3-year recurrence rates were achieved with the sorafenib–RFA combination therapy, compared with RFA alone [73]. However, a phase III trial (Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma (STORM)), which aimed to evaluate the therapeutic effect of sorafenib in preventing HCC recurrence after resection or ablation, did not find improvement in either recurrence-free survival or overall survival [74,75]. More clinical trials are warranted to reach a conclusion.
Other challenges are also associated with the combination therapy strategy: (a) the sorafenib–RFA treatment can lead to overexpression of inflammatory and growth factor signals in the entire ablated organ, which may result in tumor recurrence and accelerated repair of RFA-induced necrosis [69,76]; (b) sorafenib may result in the poor visualization of lesions [62], which can cause difficulties when outlining tumor masses during subsequent RFA; and (c) similar to PEI–RFA and TACE–RFA therapies, a standardized therapeutic protocol for this combination treatment has not yet been established.
RFA plus bevacizumab
Bevacizumab is the first anti-angiogenesis agent approved by the FDA. It is a humanized monoclonal antibody against vascular endothelial growth factor A [77]. One study demonstrated that bevacizumab is useful in preventing the rapid progression of residual HCC following RFA in a rat model [61]. Another study showed a reduction in tumor blood flow and an increased area of ablation in a mouse model of HCC, when a combination of bevacizumab and RFA was administered, compared with RFA alone [78].
In addition to sorafenib and bevacizumab, there are a number of additional targeted molecular agents against HCC currently in various clinical trial phases, such as sunitinib and linifanib [60,79,80]. However, only few of these new drugs have been reported to be used in combination with RFA so far.
RFA plus immunotherapy
Immunotherapy is a new treatment option for HCC. It aims to enhance the strength of the body's immune system against tumor-associated antigens (TAAs), and thereby induce tumor cell death. While it has attracted the attention of scientists for years, immunotherapy alone has shown no survival benefit in oncology patients so far. This may be due to the fact that most TAAs are normal self-antigens, and the origin and progression of HCC within the hepatic parenchyma results in difficulties when attempting to boost tumor-specific immunity [81,82].
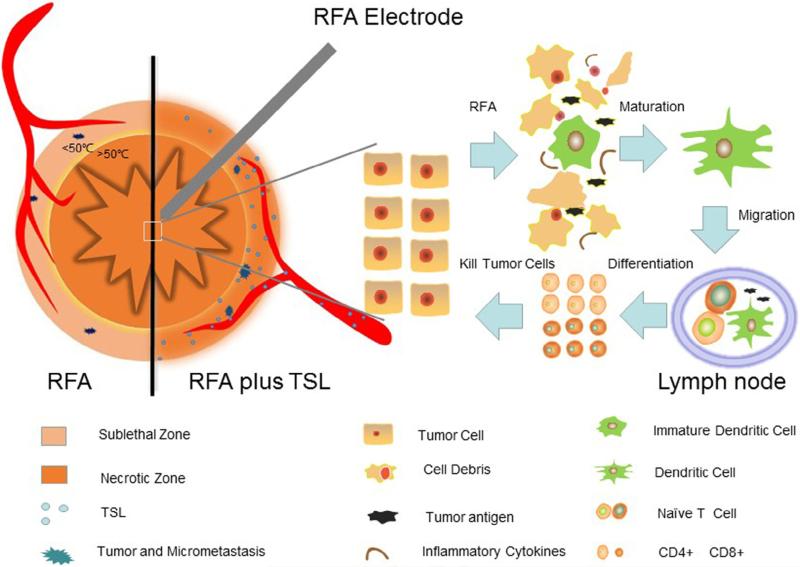
However, when used in combination with RFA, the strength of tumor-specific immunity can be improved, even though the exact mechanisms involved in this phenomenon are not yet clear [83–86]. One possible explanation is that the thermally induced necrosis can act as a permanent source of tumor antigen, which is capable of eliciting systemic anti-tumor immunity (Fig. 3). The necrosis can release antigenic material, and thereby induce infiltration of T-cells and dendritic cells (DCs). DCs play a key role in the initiation and regulation of immune responses, including antigen loading, migration and maturation [87]. Moreover, the sub-lethal zone around the necrotic zone of the RFA area can generate “danger signals” including inflammatory cytokines (such as interleukin (IL)-1, IL-6, tumor necrosis factor-α, and heat shock proteins (HSPs)), which can also stimulate the anti-tumor immune response and thereby further strengthen the immune effect with RFA [82,83]. Some studies have also found that sub-lethal thermal stress is capable of modulating tumor cell immunogenicity, and the cells become more sensitive to immune therapy [88]. Finally, RFA-enhanced immunotherapy can lead to activation of both CD4+ and CD8+ T-cells, which thus strengthens the anti-tumor immunization against potential residual tumor or metastasis following RFA [82].
Fig. 3.
Activation of the immune response and synergy of TSLs with RFA. During RFA, antigenic material is released from the treated tumor, where inflammatory cytokine and immune cells including DCs are accumulated. Antigenic material and DCs then migrate to the afferent lymph node, where tumor-specific CD4 and CD8 T-cells are developed for immune response and killing tumor cells. On the other hand, locally-delivered thermally-sensitive lipidosomes (TSLs) release rapidly chemotherapeutic drugs in sublethal area, which creates high-concentrated chemotherapeutics to eliminate micrometastases, and thereby, enlarge the necrotic zone of RFA.
Recently, studies have combined RFA with intra-tumoral injection of OK-432 or OK-432-stimulated DCs in rabbit and murine cancer models [89,90]. OK-432 is a clinical bacterial product, which can induce DC maturation [91]. The results of these studies indicated improved therapeutic outcomes with combination therapy compared with RFA alone. A more recent study reported the combined use of adoptive cellular immunotherapy with RFA in a clinical trial. Different immune-associated cells were separated and cultured from peripheral blood mononuclear cells collected from the patient before RFA and subsequently transfused back into the same patient after RFA. They concluded that the combination treatment of RFA plus immunotherapy can significantly reduce the risk of HCC recurrence [92].
RFA plus nanoparticle-mediated therapy
Nanoparticle-mediated drug delivery systems represent one of the hottest research interests in modern medicine [93]. This strategy aims to selectively or specifically deliver therapeutic agents to cancer sites using targeted nano-scaled drug carriers, which improves drug efficacy while reducing drug toxicity in other vital organs [94]. Several studies have confirmed that the combined use of nano-drugs with RFA has better efficacy in killing cancer when compared with drug alone or RFA alone. Via the potential mechanisms of RFA-generated hyperthermia or microenvironmental modification, the passive nano-drug delivery, known as the enhanced permeability and retention effect, can be strengthened when applying RFA [27,95,96]. RFA-hyperthermia can enhance the sensitivity of tumor cells to chemotherapy, while the simultaneous delivery of nano-drugs can kill residual tumors and micrometastases in the sub-lethal zone of the RFA area.
In the nano-therapeutic treatment of liver cancer, lipidosome is one of the most widely investigated nano-drug carriers [97]. One example is ThermoDox®, which is constructed with a thermal-sensitive lipidosome (TSL) delivery system (Fig. 3) [98], and has been validated in a recent phase III clinical trial (NCT00617981) in the treatment of liver cancer. ThermoDox® function is based on the structural changes of the liposome when heated to >39 °C, which can rapidly release doxorubicin into the targeted tumor region. According to the data provided by the pharmaceutical company, a better clinical outcome using ThermoDox® has been found in patients undergoing RFA when heating was prolonged for more than 45 minutes; another phase III clinical trial (NCT02112656) has been started to verify this discovery.
Prolonging liposome circulation is another well-established strategy to achieve better drug accumulation in the tumor, for example, using PEGylated doxorubicin-carrying liposomes [99]. Longer circulation time allows improved contact between drugs and tumor cells, an important factor in improving chemo-drug effectiveness. A recent study compared the therapeutic efficacies of the long-circulating PEGylated drug-liposomes and thermal-sensitive drug-liposomes in a mouse medulloblastoma model. The authors found that long-circulating liposomes resulted in longer intra-tumoral drug retention and thereby a better therapeutic effect compared with thermal-sensitive drug-liposomes when combined with RFA [100]. However, whether or not this conclusion can be adapted to treatment of HCC with other nano-drugs requires further extensive investigation.
To further enhance the therapeutic effect of such combination treatments, different therapeutics, such as quercetin, have been added into liposome complexes; with this, an enhanced approach (the so-called triple therapy of RFA, doxorubicin and quercetin) can be carried out [89]. Quercetin works by inhibiting HSP-70 expression induced by RFA, since HSP can protect tumor cells from thermal destruction [101].
Conclusion
In the current clinical practice, several therapeutic methods are available for the treatment of HCC, but the prognosis for HCC is still dismal. Different therapeutic methods have their own advantages and disadvantages, and the ideal treatment approach for advanced HCC has not yet been identified. To overcome this clinical problem, recent efforts have focused on developing multimodal treatments. One of the advances in this field is the combined use of interventional RFA with various other approaches, such as PEI, TACE, molecular targeted therapy, nanoparticle-mediated chemotherapy, and immunotherapy. A number of studies have confirmed that RFA-combined multimodal therapies can indeed improve the clinical efficacies of HCC treatment. As overviewed in this article, further efforts are needed to optimize the protocol for each of the combination therapies and thereby establish the best RFA-combined multimodal therapy strategy for the effective management of hepatic malignancies.
Acknowledgements
This study was supported by the Program for National Science and Technology Major Project of China (2013ZX10002004-001-005), National Basic Research Program of China (973 Program, 2014CB744505), US NIH RO1EBO12467 grant, Natural Science Foundation of China (81401504, 81430040) and Qianjiang Talent Program of Zhejiang Province in China (2012R10027).
Footnotes
Conflict of interest
All authors declare that there are no conflicts of interest.
References
- 1.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D, Global cancer statistics CA. Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 4.Schlachterman A, Craft WW, Jr., Hilgenfeldt E, Mitra A, Cabrera R. Current and future treatments for hepatocellular carcinoma. World J. Gastroenterol. 2015;21:8478–8491. doi: 10.3748/wjg.v21.i28.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buscarini L, Buscarini E, Di Stasi M, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur. Radiol. 2001;11:914–921. doi: 10.1007/s003300000659. [DOI] [PubMed] [Google Scholar]
- 6.Akoad ME, Pomfret EA. Surgical resection and liver transplantation for hepatocellular carcinoma. Clin. Liver Dis. 2015;19:381–399. doi: 10.1016/j.cld.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012;107:569–577. doi: 10.1038/ajg.2011.425. quiz 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin. Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 9.Shrimal A, Prasanth M, Kulkarni AV. Interventional radiological treatment of hepatocellular carcinoma: an update. Indian J. Surg. 2012;74:91–99. doi: 10.1007/s12262-011-0377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, et al. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J. Am. Coll. Surg. 2000;191:381–388. doi: 10.1016/s1072-7515(00)00700-6. [DOI] [PubMed] [Google Scholar]
- 11.Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann. Surg. 2009;249:20–25. doi: 10.1097/SLA.0b013e31818eec29. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Yan L, Wang W. Current status of multimodal & combination therapy for hepatocellular carcinoma. Indian J. Med. Res. 2012;136:391–403. [PMC free article] [PubMed] [Google Scholar]
- 14.Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur. J. Radiol. 2008;67:336–347. doi: 10.1016/j.ejrad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Kim YS, Lim HK, Rhim H, Lee MW. Ablation of hepatocellular carcinoma. Best Pract. Res. Clin. Gastroenterol. 2014;28:897–908. doi: 10.1016/j.bpg.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: current status. World J. Radiol. 2010;2:417–424. doi: 10.4329/wjr.v2.i11.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95:1931–1937. doi: 10.1002/cncr.10892. [DOI] [PubMed] [Google Scholar]
- 18.Rhim H, Goldberg SN, Dodd GD, 3rd, Solbiati L, Lim HK, Tonolini M, et al. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;21:S17–S35. doi: 10.1148/radiographics.21.suppl_1.g01oc11s17. discussion S36–19. [DOI] [PubMed] [Google Scholar]
- 19.Park MJ, Kim YS, Rhim H, Lim HK, Lee MW, Choi D. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J. Vasc. Interv. Radiol. 2011;22:771–779. doi: 10.1016/j.jvir.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Woo S, Lee JM, Yoon JH, Joo I, Kim SH, Lee JY, et al. Small- and mediumsized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system-mid-term results. Radiology. 2013;268:589–600. doi: 10.1148/radiol.13121736. [DOI] [PubMed] [Google Scholar]
- 21.Schumacher PA, Powell JJ, MacNeill AJ, Buczkowski AK, Erb SR, Ho SG, et al. Multimodal therapy for hepatocellular carcinoma: a complementary approach to liver transplantation. Ann. Hepatol. 2010;9:23–32. [PubMed] [Google Scholar]
- 22.Sturm JW, Keese M. Multimodal treatment of hepatocellular carcinoma (HCC) Onkologie. 2004;27:294–303. doi: 10.1159/000077982. [DOI] [PubMed] [Google Scholar]
- 23.Rossi S, Fornari F, Buscarini L. Percutaneous ultrasound-guided radiofrequency electrocautery for the treatment of small hepatocellular carcinoma. J. Interv. Radiol. 1993;8:97–103. [Google Scholar]
- 24.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 25.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed M, Brace CL, Lee FT, Jr., Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat. Rev. Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg SN, Gazelle GS, Halpern EF, Rittman WJ, Mueller PR, Rosenthal DI. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad. Radiol. 1996;3:212–218. doi: 10.1016/s1076-6332(96)80443-0. [DOI] [PubMed] [Google Scholar]
- 29.Mertyna P, Hines-Peralta A, Liu ZJ, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: variability in heat sensitivity in tumors and tissues. J. Vasc. Interv. Radiol. 2007;18:647–654. doi: 10.1016/j.jvir.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Han G, Wang Y, Hu X, Li Z, Chen L, et al. Radiofrequency heat-enhanced chemotherapy for breast cancer: towards interventional molecular image-guided chemotherapy. Theranostics. 2014;4:1145–1152. doi: 10.7150/thno.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Le T, Wu X, Wang H, Zhang T, Meng Y, et al. Intrabiliary RF heat-enhanced local chemotherapy of a cholangiocarcinoma cell line: monitoring with dual-modality imaging – preclinical study. Radiology. 2014;270:400–408. doi: 10.1148/radiol.13130866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101–108. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- 33.Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, et al. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J. Hepatol. 2005;43:458–464. doi: 10.1016/j.jhep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 34.Kwon JH. Is percutaneous ethanol injection therapy still effective for hepatocellular carcinoma in the era of radiofrequency ablation? Gut Liver. 2010;4(Suppl. 1):S105–S112. doi: 10.5009/gnl.2010.4.S1.S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe S, Kurokohchi K, Masaki T, Miyauchi Y, Funaki T, Inoue H, et al. Enlargement of thermal ablation zone by the combination of ethanol injection and radiofrequency ablation in excised bovine liver. Int. J. Oncol. 2004;24:279–284. [PubMed] [Google Scholar]
- 36.Goldberg SN, Kruskal JB, Oliver BS, Clouse ME, Gazelle GS. Percutaneous tumor ablation: increased coagulation by combining radio-frequency ablation and ethanol instillation in a rat breast tumor model. Radiology. 2000;217:827–831. doi: 10.1148/radiology.217.3.r00dc27827. [DOI] [PubMed] [Google Scholar]
- 37.Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Funaki T, Arima K, et al. Combined use of percutaneous ethanol injection and radiofrequency ablation for the effective treatment of hepatocellular carcinoma. Int. J. Oncol. 2002;21:841–846. doi: 10.3892/ijo.21.4.841. [DOI] [PubMed] [Google Scholar]
- 38.Sakr AA, Saleh AA, Moeaty AA, Moeaty AA. The combined effect of radiofrequency and ethanol ablation in the management of large hepatocellular carcinoma. Eur. J. Radiol. 2005;54:418–425. doi: 10.1016/j.ejrad.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Miyauchi Y, Himoto T, et al. Comparison between combination therapy of percutaneous ethanol injection and radiofrequency ablation and radiofrequency ablation alone for patients with hepatocellular carcinoma. World J. Gastroenterol. 2005;11:1426–1432. doi: 10.3748/wjg.v11.i10.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong SN, Lin CJ, Lin CC, Chen WT, Cua IH, Lin SM. Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. AJR Am. J. Roentgenol. 2008;190:W187–W195. doi: 10.2214/AJR.07.2537. [DOI] [PubMed] [Google Scholar]
- 41.Huang G, Lin M, Xie X, Liu B, Xu Z, Lencioni R, et al. Combined radiofrequency ablation and ethanol injection with a multipronged needle for the treatment of medium and large hepatocellular carcinoma. Eur. Radiol. 2014;24:1565–1571. doi: 10.1007/s00330-014-3151-8. [DOI] [PubMed] [Google Scholar]
- 42.Lin JW, Lin CC, Chen WT, Lin SM. Combining radiofrequency ablation and ethanol injection may achieve comparable long-term outcomes in larger hepatocellular carcinoma (3.1-4 cm) and in high-risk locations. Kaohsiung J. Med. Sci. 2014;30:396–401. doi: 10.1016/j.kjms.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Azab M, Zaki S, El-Shetey AG, Abdel-Moty MF, Alnoomani NM, Gomaa AA, et al. Radiofrequency ablation combined with percutaneous ethanol injection in patients with hepatocellular carcinoma. Arab J. Gastroenterol. 2011;12:113–118. doi: 10.1016/j.ajg.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Vallone P, Catalano O, Izzo F, Siani A. Combined ethanol injection therapy and radiofrequency ablation therapy in percutaneous treatment of hepatocellular carcinoma larger than 4 cm. Cardiovasc. Intervent. Radiol. 2006;29:544–551. doi: 10.1007/s00270-005-0173-2. [DOI] [PubMed] [Google Scholar]
- 45.Zhang YJ, Liang HH, Chen MS, Guo RP, Li JQ, Zheng Y, et al. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randomized trial. Radiology. 2007;244:599–607. doi: 10.1148/radiol.2442060826. [DOI] [PubMed] [Google Scholar]
- 46.Kurokohchi K, Masaki T, Watanabe S, Nakai S, Deguchi A, Morishita A, et al. Time-lag performance of radiofrequency ablation after percutaneous ethanol injection for the treatment of hepatocellular carcinoma. Int. J. Oncol. 2006;28:971–976. [PubMed] [Google Scholar]
- 47.Kurokohchi K, Masaki T, Miyauchi Y, Hosomi N, Yoneyama H, Yoshida S, et al. Efficacy of combination therapies of percutaneous or laparoscopic ethanol-lipiodol injection and radiofrequency ablation. Int. J. Oncol. 2004;25:1737–1743. doi: 10.3892/ijo.25.6.1737. [DOI] [PubMed] [Google Scholar]
- 48.Liao M, Huang J, Zhang T, Wu H. Transarterial chemoembolization in combination with local therapies for hepatocellular carcinoma: a meta-analysis. PLoS ONE. 2013;8:e68453. doi: 10.1371/journal.pone.0068453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogl TJ, Naguib NN, Nour-Eldin NE, Rao P, Emami AH, Zangos S, et al. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur. J. Radiol. 2009;72:505–516. doi: 10.1016/j.ejrad.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Pleguezuelo M, Marelli L, Misseri M, Germani G, Calvaruso V, Xiruochakis E, et al. TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev. Anticancer Ther. 2008;8:1623–1641. doi: 10.1586/14737140.8.10.1623. [DOI] [PubMed] [Google Scholar]
- 51.Seki T, Tamai T, Nakagawa T, Imamura M, Nishimura A, Yamashiki N, et al. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer. 2000;89:1245–1251. [PubMed] [Google Scholar]
- 52.Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J. Surg. 2004;28:376–381. doi: 10.1007/s00268-003-7308-x. [DOI] [PubMed] [Google Scholar]
- 53.Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer. 1994;73:2259–2267. doi: 10.1002/1097-0142(19940501)73:9<2259::aid-cncr2820730905>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 54.Zhao M, Wang JP, Pan CC, Li W, Huang ZL, Zhang L, et al. CT-guided radiofrequency ablation after with transarterial chemoembolization in treating unresectable hepatocellular carcinoma with long overall survival improvement. Eur. J. Radiol. 2012;81:2717–2725. doi: 10.1016/j.ejrad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 55.Lee MW, Kim YJ, Park SW, Hwang JH, Jung SI, Jeon HJ, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma invisible on both ultrasonography and unenhanced CT: a preliminary study of combined treatment with transarterial chemoembolisation. Br. J. Radiol. 2009;82:908–915. doi: 10.1259/bjr/55877882. [DOI] [PubMed] [Google Scholar]
- 56.Llovet JM, Vilana R, Bru C, Bianchi L, Salmeron JM, Boix L, et al. Clinic liver cancer, increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 57.Dong W, Zhang T, Wang ZG, Liu H. Clinical outcome of small hepatocellular carcinoma after different treatments: a meta-analysis. World J. Gastroenterol. 2014;20:10174–10182. doi: 10.3748/wjg.v20.i29.10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J. Gastroenterol. 2013;19:3872–3882. doi: 10.3748/wjg.v19.i24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choe WH, Kim YJ, Park HS, Park SW, Kim JH, Kwon SY. Short-term interval combined chemoembolization and radiofrequency ablation for hepatocellular carcinoma. World J. Gastroenterol. 2014;20:12588–12594. doi: 10.3748/wjg.v20.i35.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu AX. New agents on the horizon in hepatocellular carcinoma. Ther. Adv. Med. Oncol. 2013;5:41–50. doi: 10.1177/1758834012458480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kong J, Kong J, Pan B, Ke S, Dong S, Li X, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1alpha/VEGFA. PLoS ONE. 2012;7:e37266. doi: 10.1371/journal.pone.0037266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haydar AA, Mukherji D, Faraj W, Khalifeh M, Taslakian B, Yehia ZA, et al. Challenges in combining antiangiogenic therapy with transarterial chemoembolization for hepatocellular carcinoma. Gastrointest. Cancer Res. 2014;7:98–102. [PMC free article] [PubMed] [Google Scholar]
- 63.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 64.McDermott U, Sharma SV, Dowell L, Greninger P, Montagut C, Lamb J, et al. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19936–19941. doi: 10.1073/pnas.0707498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tai WT, Cheng AL, Shiau CW, Huang HP, Huang JW, Chen PJ, et al. Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J. Hepatol. 2011;55:1041–1048. doi: 10.1016/j.jhep.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 66.Zhang CZ, Wang XD, Wang HW, Cai Y, Chao LQ. Sorafenib inhibits liver cancer growth by decreasing mTOR, AKT, and PI3K expression. J. BUON. 2015;20:218–222. [PubMed] [Google Scholar]
- 67.Chen KW, Ou TM, Hsu CW, Horng CT, Lee CC, Tsai YY, et al. Current systemic treatment of hepatocellular carcinoma: a review of the literature. World J. Hepatol. 2015;7:1412–1420. doi: 10.4254/wjh.v7.i10.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hakime A, Hines-Peralta A, Peddi H, Atkins MB, Sukhatme VP, Signoretti S, et al. Combination of radiofrequency ablation with antiangiogenic therapy for tumor ablation efficacy: study in mice. Radiology. 2007;244:464–470. doi: 10.1148/radiol.2442061005. [DOI] [PubMed] [Google Scholar]
- 69.Mertens JC, Martin IV, Schmitt J, Frei P, Bruners P, Herweg C, et al. Multikinase inhibitor sorafenib transiently promotes necrosis after radiofrequency ablation in rat liver but activates growth signals. Eur. J. Radiol. 2012;81:1601–1606. doi: 10.1016/j.ejrad.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 70.Eros de Bethlenfalva-Hora C, Mertens JC, Piguet AC, Kettenbach J, Schmitt J, Terracciano L, et al. Radiofrequency ablation suppresses distant tumour growth in a novel rat model of multifocal hepatocellular carcinoma. Clin. Sci. 2014;126:243–252. doi: 10.1042/CS20130089. [DOI] [PubMed] [Google Scholar]
- 71.Fukuda H, Numata K, Moriya S, Shimoyama Y, Ishii T, Nozaki A, et al. Hepatocellular carcinoma: concomitant sorafenib promotes necrosis after radiofrequency ablation –propensity score matching analysis. Radiology. 2014;272:598–604. doi: 10.1148/radiol.14131640. [DOI] [PubMed] [Google Scholar]
- 72.Kan X, Jing Y, Wan QY, Pan JC, Han M, Yang Y, et al. Sorafenib combined with percutaneous radiofrequency ablation for the treatment of medium-sized hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2015;19:247–255. [PubMed] [Google Scholar]
- 73.Feng X, Xu R, Du X, Dou K, Qin X, Xu J, et al. Combination therapy with sorafenib and radiofrequency ablation for BCLC Stage 0-B1 hepatocellular carcinoma: a multicenter retrospective cohort study. Am. J. Gastroenterol. 2014;109:1891–1899. doi: 10.1038/ajg.2014.343. [DOI] [PubMed] [Google Scholar]
- 74.Facciorusso A, Muscatiello N, Di Leo A, Barone M. Combination therapy with sorafenib and radiofrequency ablation for hepatocellular carcinoma: a glimmer of light after the storm trial? Am. J. Gastroenterol. 2015;110:770–771. doi: 10.1038/ajg.2015.80. [DOI] [PubMed] [Google Scholar]
- 75.Feng X, Ma K. Response to Facciorusso et al. Am. J. Gastroenterol. 2015;110:771–773. doi: 10.1038/ajg.2015.83. [DOI] [PubMed] [Google Scholar]
- 76.Rozenblum N, Zeira E, Bulvik B, Gourevitch S, Yotvat H, Galun E, et al. Radiofrequency ablation: inflammatory changes in the periablative zone can induce global organ effects, including liver regeneration. Radiology. 2015:141918. doi: 10.1148/radiol.15141918. [DOI] [PubMed] [Google Scholar]
- 77.Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134:1296–1310. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thaker AA, Razjouyan F, Woods DL, Haemmerich D, Sekhar K, Wood BJ, et al. Combination therapy of radiofrequency ablation and bevacizumab monitored with power Doppler ultrasound in a murine model of hepatocellular carcinoma. Int. J. Hyperthermia. 2012;28:766–775. doi: 10.3109/02656736.2012.724517. [DOI] [PubMed] [Google Scholar]
- 79.Moussa M, Goldberg SN, Kumar G, Sawant RR, Levchenko T, Torchilin V, et al. Radiofrequency ablation-induced upregulation of hypoxia-inducible factor-1alpha can be suppressed with adjuvant bortezomib or liposomal chemotherapy. J. Vasc. Interv. Radiol. 2014;25:1972–1982. doi: 10.1016/j.jvir.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ch'ang HJ. Optimal combination of antiangiogenic therapy for hepatocellular carcinoma. World J. Hepatol. 2015;7:2029–2040. doi: 10.4254/wjh.v7.i16.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miamen AG, Dong H, Roberts LR. Immunotherapeutic approaches to hepatocellular carcinoma treatment. Liver Cancer. 2012;1:226–237. doi: 10.1159/000343837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fagnoni FF, Zerbini A, Pelosi G, Missale G. Combination of radiofrequency ablation and immunotherapy. Front. Biosci. 2008;13:369–381. doi: 10.2741/2686. [DOI] [PubMed] [Google Scholar]
- 83.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 84.Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448–1457. doi: 10.1002/hep.26153. [DOI] [PubMed] [Google Scholar]
- 85.Wissniowski TT, Hansler J, Neureiter D, Frieser M, Schaber S, Esslinger B, et al. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63:6496–6500. [PubMed] [Google Scholar]
- 86.Goldberg SN. Science to practice: can we expand focal interventional oncologic ablation treatments into an effective systemic therapy? Radiology. 2013;267:321–323. doi: 10.1148/radiol.13130140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 88.Gameiro SR, Higgins JP, Dreher MR, Woods DL, Reddy G, Wood BJ, et al. Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression. PLoS ONE. 2013;8:e70417. doi: 10.1371/journal.pone.0070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kageyama K, Yamamoto A, Okuma T, Hamamoto S, Takeshita T, Sakai Y, et al. Radiofrequency ablation of liver tumors in combination with local OK-432 injection prolongs survival and suppresses distant tumor growth in the rabbit model with intra- and extrahepatic VX2 tumors. Cardiovasc. Intervent. Radiol. 2013;36:1383–1392. doi: 10.1007/s00270-013-0650-y. [DOI] [PubMed] [Google Scholar]
- 90.Nakagawa H, Mizukoshi E, Iida N, Terashima T, Kitahara M, Marukawa Y, et al. In vivo immunological antitumor effect of OK-432-stimulated dendritic cell transfer after radiofrequency ablation. Cancer Immunol. Immunother. 2014;63:347–356. doi: 10.1007/s00262-013-1514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakahara S, Tsunoda T, Baba T, Asabe S, Tahara H. Dendritic cells stimulated with a bacterial product, OK-432, efficiently induce cytotoxic T lymphocytes specific to tumor rejection peptide. Cancer Res. 2003;63:4112–4118. [PubMed] [Google Scholar]
- 92.Cui J, Wang N, Zhao H, Jin H, Wang G, Niu C, et al. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int. J. Cancer. 2014;134:342–351. doi: 10.1002/ijc.28372. [DOI] [PubMed] [Google Scholar]
- 93.Saenz del Burgo L, Pedraz JL, Orive G. Advanced nanovehicles for cancer management. Drug Discov. Today. 2014;19:1659–1670. doi: 10.1016/j.drudis.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 94.Yang X, editor. Nanotechnology in Modern Medical Imaging and Interventions. Nova Science Publishers, Inc.; Hauppauge, NY: 2013. [Google Scholar]
- 95.Moussa M, Goldberg SN, Tasawwar B, Sawant RR, Levchenko T, Kumar G, et al. Adjuvant liposomal doxorubicin markedly affects radiofrequency ablation-induced effects on periablational microvasculature. J. Vasc. Interv. Radiol. 2013;24:1021–1033. doi: 10.1016/j.jvir.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moussa M, Goldberg SN, Kumar G, Sawant RR, Levchenko T, Torchilin VP, et al. Nanodrug-enhanced radiofrequency tumor ablation: effect of micellar or liposomal carrier on drug delivery and treatment efficacy. PLoS ONE. 2014;9:e102727. doi: 10.1371/journal.pone.0102727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahmed M, Moussa M, Goldberg SN. Synergy in cancer treatment between liposomal chemotherapeutics and thermal ablation. Chem. Phys. Lipids. 2012;165:424–437. doi: 10.1016/j.chemphyslip.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.May JP, Li SD. Hyperthermia-induced drug targeting. Expert Opin. Drug Deliv. 2013;10:511–527. doi: 10.1517/17425247.2013.758631. [DOI] [PubMed] [Google Scholar]
- 99.Barenholz Y. Doxil(R) –the first FDA-approved nano-drug: lessons learned. J. Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 100.Andriyanov AV, Koren E, Barenholz Y, Goldberg SN. Therapeutic efficacy of combining pegylated liposomal doxorubicin and radiofrequency (RF) ablation: comparison between slow-drug-releasing, non-thermosensitive and fast-drug-releasing, thermosensitive nano-liposomes. PLoS ONE. 2014;9:e92555. doi: 10.1371/journal.pone.0092555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang W, Ahmed M, Tasawwar B, Levchenko T, Sawant RR, Torchilin V, et al. Combination radiofrequency (RF) ablation and IV liposomal heat shock protein suppression: reduced tumor growth and increased animal endpoint survival in a small animal tumor model. J. Control Release. 2012;160:239–244. doi: 10.1016/j.jconrel.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]