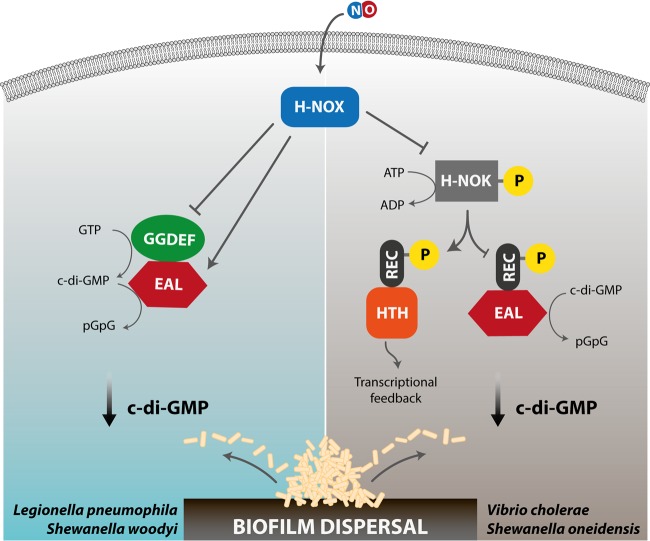
FIG 3.
Nitric oxide-induced signaling events mediated by the heme-based sensor domain H-NOX. The binding of NO to the heme moiety of H-NOX may result in biofilm dispersal following different signaling cascades in different bacterial species harboring this protein domain. Left, in Legionella pneumophila or Shewanella woodyi, the NO derivative of the H-NOX protein interacts with the GGDEF-EAL (HaCE) protein, thus lowering c-di-GMP levels by stimulation of the PDE activity of the HaCE EAL domain. Right, in Vibrio cholerae or Shewanella oneidensis, interaction of the NO-bound H-NOX domain with a coupled histidine kinase (H-NOK) controls the phosphorylation activity of the kinase. Specific phosphorylation events lead to a decrease in c-di-GMP levels, either by stimulating the hydrolysis of c-di-GMP to pGpG by a cognate PDE (REC-EAL) via the fused REC domain or by controlling the transcriptional response through a dedicated transcription regulator (REC-HTH).