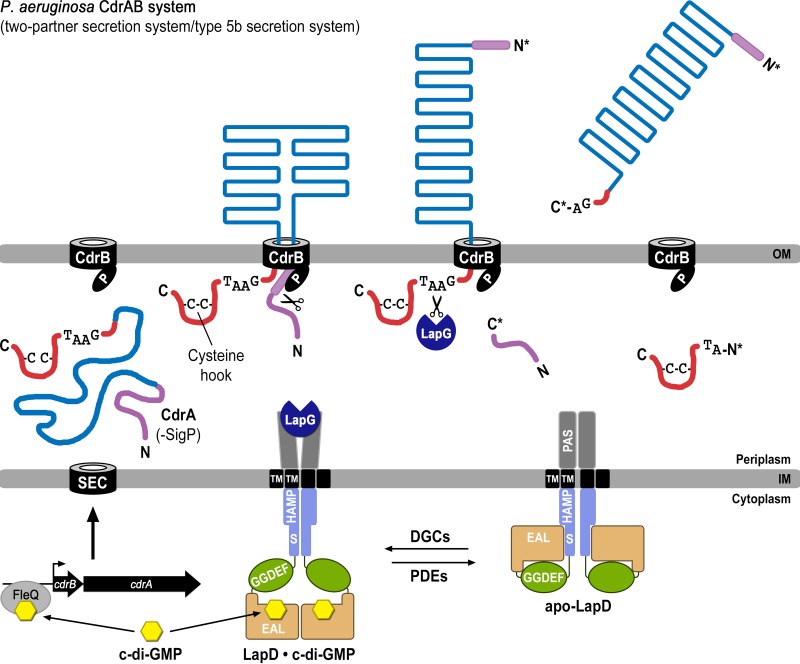
FIG 7.
Model for two-tier regulation of CdrA via c-di-GMP. Transcription of the cdrAB operon (bottom left) is initiated when c-di-GMP (yellow) binds the transcriptional regulator FleQ. Elevated c-di-GMP levels also result in second-messenger binding to the EAL domain of LapD, which in turn sequesters periplasmic LapG. Together these events allow CdrA to be secreted to and anchored within the outer membrane via its cognate outer membrane transporter CdrB. The N terminus of CdrA (purple) binds to the PORTA domain of CdrB and is processed by an unknown proteolytic activity, acting in the periplasm (as shown), within the transporter, or at the cell surface. When c-di-GMP levels are lowered by phosphodiesterases (PDEs), LapD adopts the autoinhibited state (bottom right), releasing LapG into the periplasm, where it cleaves the C-terminal tail of CdrA (red) at the TAAG cleavage site and releases this adhesin into the extracellular space, promoting cellular disaggregation.