FIG 5.
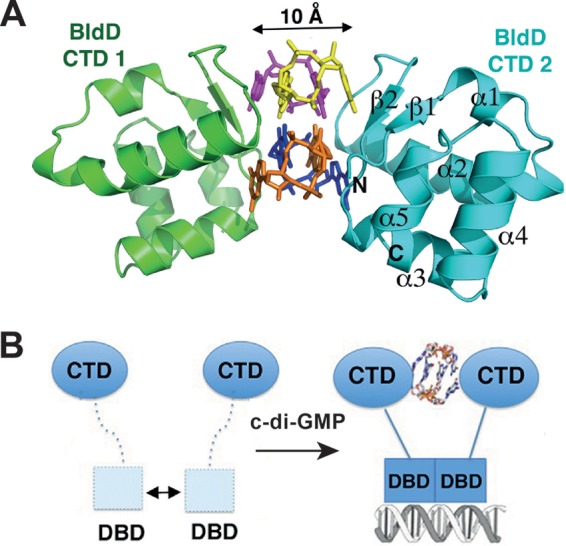
BldD in Streptomyces. A key developmental regulator is regulated by c-di-GMP and reveals a novel mode of c-di-GMP binding. (A) Ribbon diagram of the C-terminal domain (CTD) of BldD in complex with c-di-GMP. The BldD-CTD complex uses a previously unseen mode of c-di-GMP binding in which two noninteracting CTDs separated by 10 Å are glued together by a c-di-GMP tetramer composed of two intercalated c-di-GMP dimers. The four c-di-GMP molecules are shown in different colors. (B) Schematic representation of c-di-GMP-activated BldD binding to DNA. The formation of a c-di-GMP-linked BldD-CTD dimer enables the N-terminal DNA-binding domain (DBD) to optimally dimerize and to effectively bind to target DNA, leading to repression of the BldD regulon. A weak interaction between DBDs in the absence of c-di-GMP is shown by the double-headed arrow. The figure was adapted from reference 66 and is used here with permission from the publisher.
