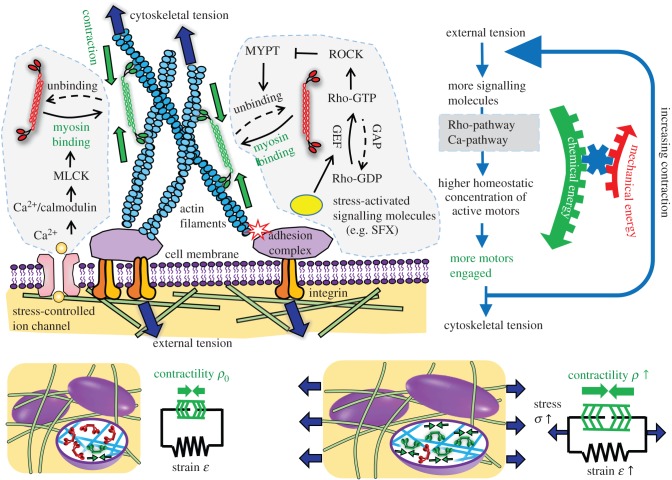
Figure 1.
Schematic depiction of the chemo-mechanical coupling and stress-dependent feedback mechanisms in our model. Both Rho-ROCK and Ca-pathways control stress-dependent myosin motor recruitment and binding with the cytoskeleton. Under homeostatic conditions, the stress-fibre network applies tensile forces to the molecular complex at the focal adhesions [22], which trigger a variety of biochemical processes. One of these events is the conformation change of Vinculin and p130Cas, exposing binding sites of SFKs [23,24]. SFKs act on Rho-GTPases by controlling the activity of GEFs and GAPs, and the increased activity of Rho promotes ROCK-mediated phosphorylation of MYPT, which, ultimately, downregulates motor unbinding [25]. The Ca2+ pathway regulates the rapid binding of motors to the cytoskeleton. The cytoskeleton transmits tensile force into the cell membrane and activates stress-sensitive ion channels [26]. This process induces Ca2+ flux into the cytoplasm and promotes motor binding. The main outcome of these stress-dependent signalling pathways is that motors switch from inactive states (red) to active states (green), which causes an increase in the density of force dipoles and alignment in the direction of applied stress. Collectively, these force dipoles produce polarized contraction in cell-populated ECMs. This process is captured by the two-element model with an active element (green) that acts in parallel with the passive element that represents cell stiffness. Applied stress σ causes the contractility of the active element ρ to increase. (Online version in colour.)