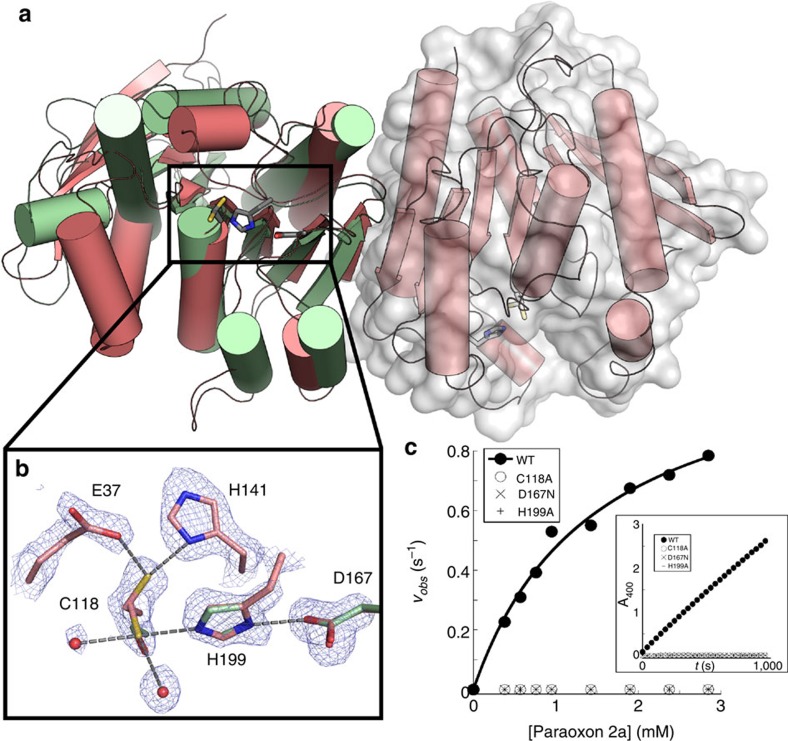
Figure 5. A triad is the catalytic feature of the α/β-hydrolase fold of the triesterase P91.
(a) The P91 structure (red) is aligned with DLH (green), a well-characterized α/β hydrolase fold (PDB ID: 1ZIC43). (b) Active site superposition with DLH reveals the catalytic triad of P91 consisting of C118, D167 and H199. E37 and H141 stabilize an alternative cysteine conformation. (c) Site-directed knockdown mutagenesis of the residues in the triad compromises P91's PTE activity (vobs=v/[E0]) (shown by Michaelis–Menten plot and time-course measurements using ∼1 mM of substrate (framed)). Conditions: activity towards paraoxon 2a measured at 25 °C (100 mM Tris-HCl, 150 mM NaCl pH 8.0).