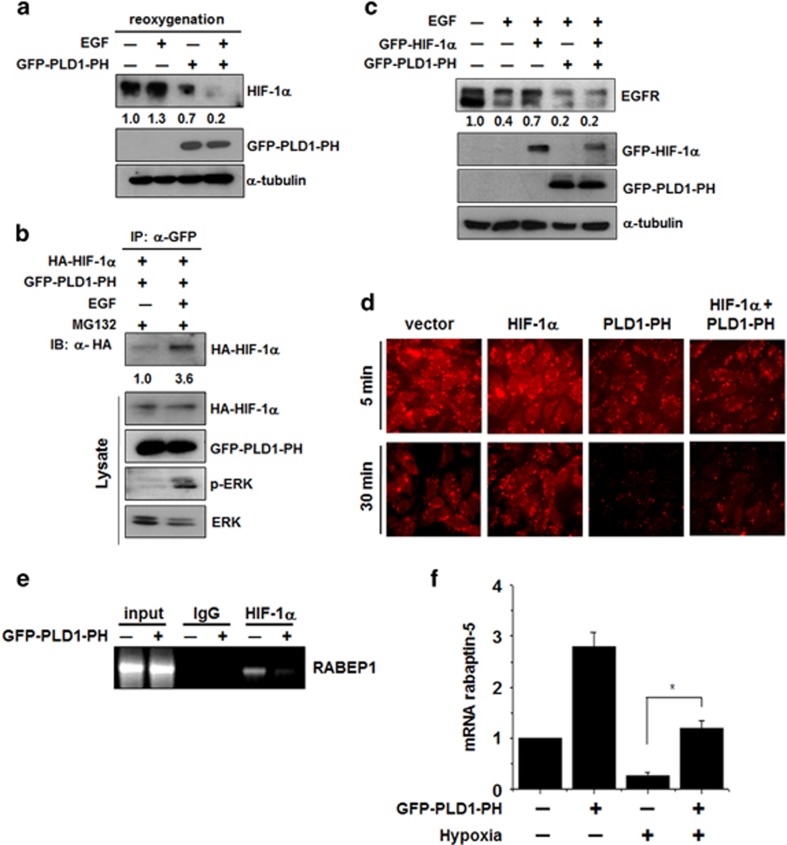
Figure 4.
PH-domain of PLD1 is crucial for EGFR endocytosis by increasing the level of rabaptin-5. (a) HEK293 cells transfected with GFP–PLD1-PH and incubated under hypoxia. After hypoxia for 4 h, the cells were reoxygenized with treatment of EGF (100 ng ml−1) for 30 min. The values were normalized to that of α-tubulin and expressed as a fold of the control. (b) HEK293 cells were cotransfected with the indicated constructs, then exposed to EGF (100 ng ml−1) for 30 min in the presence of MG132. The lysates were immunoprecipitated and immunoblotted with the indicated antibodies. (c) Immunoblot assay of EGFR under the indicated condition. The values were normalized to that of α-tubulin and expressed as a fold of the control. (d) HEK293 cells were cotransfected with GFP–HIF-1α and GFP–PLD1-PH. After 24 h of serum starvation, the cells were treated with Alexa Fluor 555–EGF (20 ng ml−1) for the indicated times, fixed and examined by fluorescence microscopy. Data are representative of three independent experiments. (e) ChIP assay for the binding of HIF-1α to the RABEP1 promoter in HEK293 cells transfected with PLD1-PH. (f) Quantitative RT–PCR assay of rabaptin-5 mRNA in HEK293 cells transfected with GFP–PLD1-PH and incubated under hypoxia. *P<0.05, results are shown as the mean±s.d. of the three independent experiments. EGFR, epidermal growth factor receptor; HIF-1α, hypoxia-inducible factor-1α GFP, green fluorescent protein; PH, pleckstrin homology; PLD, phospholipase D.