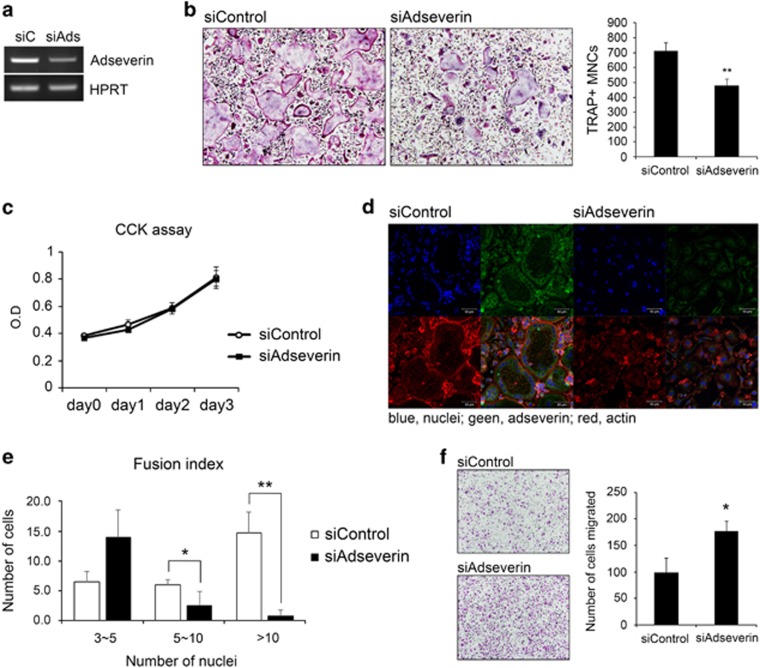
Figure 2.
Adseverin depletion impairs osteoclast differentiation and actin ring formation. (a) Bone marrow-derived macrophages (BMMs) were transfected with 30 nM of control or adseverin-specific small interfering RNA (siRNA) and cultured for 2 days with 120 ng ml−1 receptor activator of nuclear factor-κB ligand (RANKL) and 30 ng ml−1 macrophage-colony-stimulating factor (M-CSF). Adseverin mRNA expression was then analyzed using reverse transcription-polymerase chain reaction (RT-PCR). (b) siRNA-transfected BMMs were cultured with M-CSF and RANKL for 4 days. Cells were fixed and then stained for tartrate-resistant acid phosphatase (TRAP). Images were captured using a light microscope (magnification × 100), and TRAP-positive multinucleated cells (MNCs) were counted. (c) siRNA-transfected BMMs were cultured in 96-well plates. CCK assays were performed on days 0, 1, 2 and 3 post-RANKL treatment. (d) BMMs were cultured with 30 ng ml−1 M-CSF and 120 ng ml−1 RANKL for 4 days. The actin ring was labeled with rhodamine-phalloidin (red), and cells were also immunostained using anti-adseverin-FITC (fluorescein isothiocyanate) antibodies. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). (e) As in d, fixed cells were stained with DAPI and rhodamine-phalloidin and then counted for the number of nuclei per osteoclast. (f) BMMs were cultured for 2 days with M-CSF and RANKL, and migration assays were performed for 16 h using transwell plates. The data are presented as the means±s.d. *P<0.05; **P<0.005.