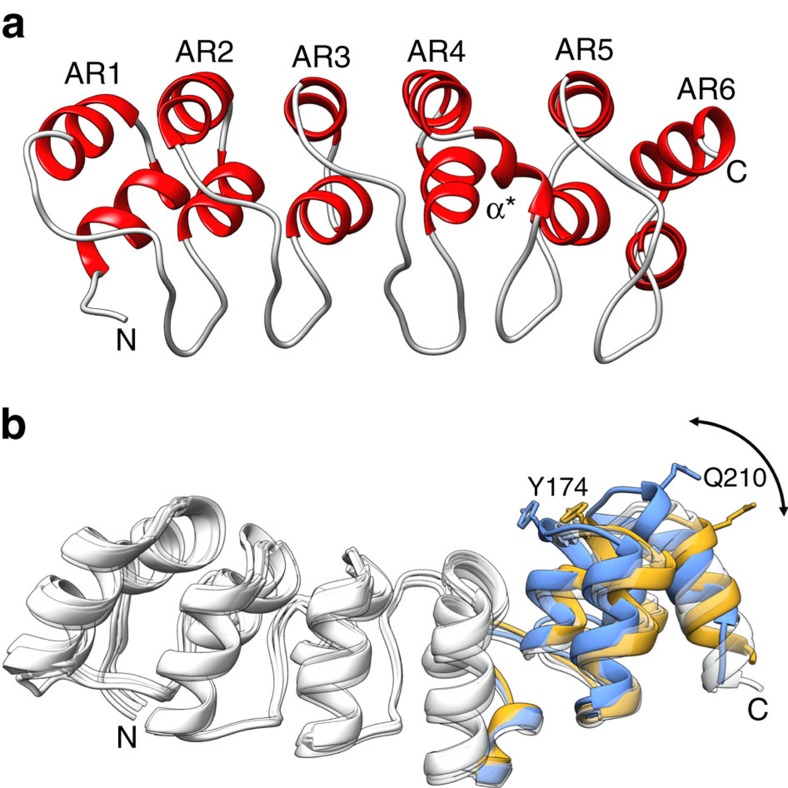
Figure 4. Structure of the endopeptidase self-protection protein Bd3460.
(a) Sequential ankyrin repeats (AR) form the core of Bd3460, with a crossover helix (α*) between AR4/5; the AR4:AR5 packing differs from the other repeats, leading to a ‘4+2' arrangement. (b) View ∼90° from that in a, demonstrating relative flexation between the extended (orange, unbound chain A) and endopeptidase target-complexed (blue) forms of Bd3460, residues Y174 and Q210 represented in stick form to guide interpretation (AR1-4 conformation common to all forms, coloured white; chains B–E of unbound form represent states of intermediate conformation and are rendered transparent for clarity).