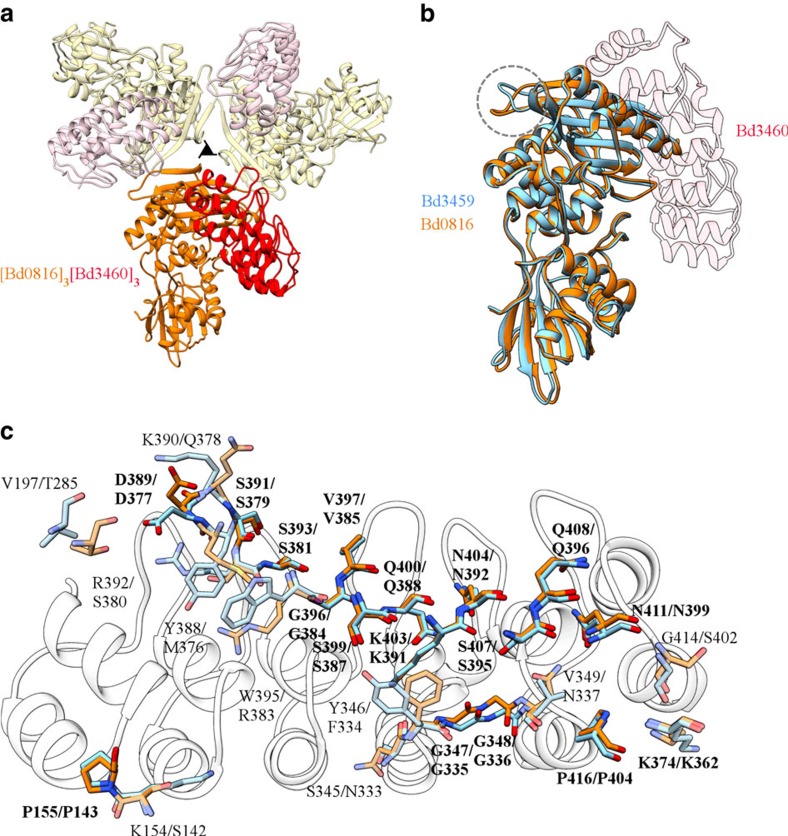
Figure 6. Complexation of the Bd3460 self-protection protein with the second endopeptidase partner Bd0816.
(a) Heterohexameric Bd08163:Bd34603 complex, with a single pair (Bd0816, orange; Bd3460, red) in bold and remainder of hexamer transparent (Bd0816, yellow; Bd3460, pink). (b) Superimposition (using Bd3460, transparent) of the related Bd3459 and Bd0816 endopeptidases (blue and orange, respectively). The largest structural difference between the two targets forms part of the Bd0816 trimer interface (circled)—generally the folds show high equivalence. (c) Bd0816/Bd3459: Bd3460 interaction face comparison; Bd3459 (blue) and Bd0816 (orange) display a common, conserved interface (labels in bold type, standard stick form; located largely at the final transpeptidase domain α helix highlighted in Fig. 5b) at the core of the interaction, surrounded by a less conserved ‘halo' of variant interacting residues (labels in normal type, transparent stick form).