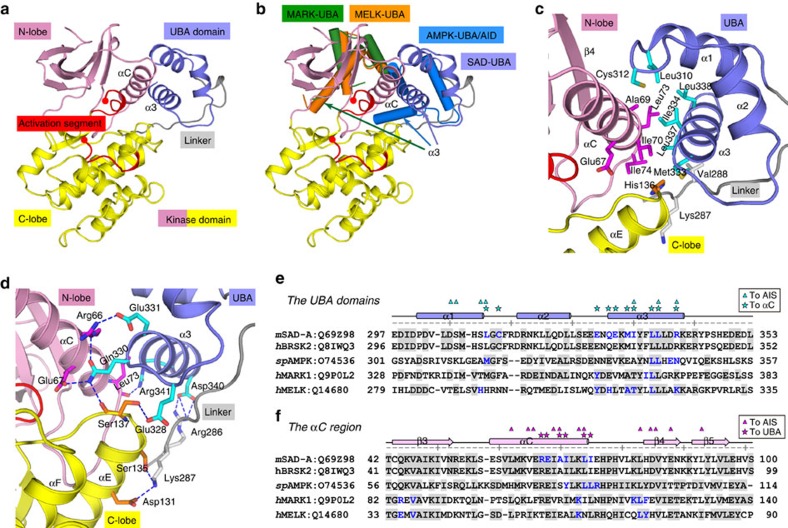
Figure 2. Distinct binding modes between SAD-A kinase and UBA domains.
(a) Overall structure of SAD-A KD-UBA. The color scheme follows that in Fig. 1a. (b) Comparison of the KD-UBA structures from SAD-A, AMPK (PDB code: 3H4J), MARK1 (2HAK) and MELK (4IXP) upon superposition of kinase C-lobes. For clarity, only the UBA domains from AMPK, MARK1 and MELK are displayed. (c,d) Close-up views of the KD-UBA interface. The interacting residues from UBA are highlighted as cyan sticks, and those from the kinase N- and C-lobes are shown as magenta and orange sticks, respectively. Blue dashed lines represent the polar interactions. (e,f) Structure-based sequence alignments of the UBA domains and the αC regions from SAD-A, BRSK2, AMPK, MARK1 and MELK. Residues involved in respective KD–UBA interactions are highlighted in blue. Residues at the KD-UBA interface of SAD-A are also indicated by asterisks, and the AIS-interacting residues are indicated by triangles. The code following each protein name is the corresponding UniProt ID. m, mouse; h, human; sp, Schizosaccharomyces pombe.