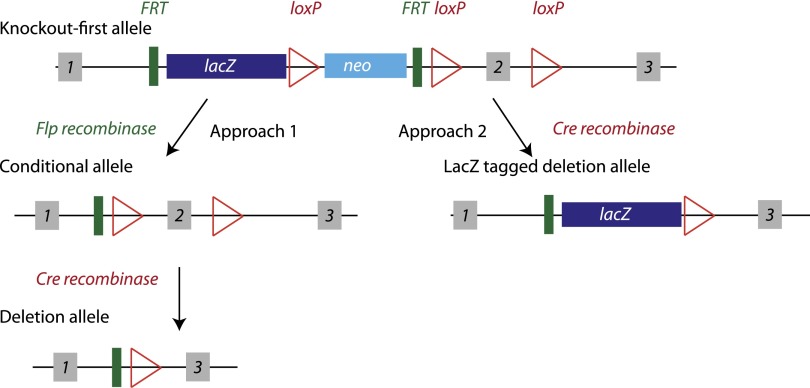
Figure 1.
Schematic representation of the commonly used “knockout-first” allele from IKMC. Adapted from Skarnes et al. (8). The targeting allele contains a cassette with the lacZ gene and a promoter-driven neomycin resistance gene (neo), flanked by Flp recombinase target (FRT) sites. This cassette is inserted into an intron of the targeted gene to disrupt endogenous gene expression and allow lacZ reporter expression under control of the endogenous gene promoter. A critical exon of the targeted gene is also flanked by loxP sites. There is also a loxP site between lacZ and neo. There are two recommended approaches for generating animals. 1) Breed first with a Flp-recombinase mouse, removing both the lacZ and neo between the FRT sites. This conditional allele can then be bred with a Cre-recombinase mouse to remove the flanked exon between the loxP sites, creating a deletion allele with no reporter. 2) Breed with a Cre-recombinase mouse first, removing both the neo and the loxP-flanked exon, generating a reporter-tagged deletion allele. The first approach is typically followed in generation of knockout animals, necessitating two generations of breeding with recombinase mice. Breeding with Flp-recombinase or Cre-recombinase mice in the C57BL/6N background will produce a deletion allele in an isogenic background. Breeding with other strains will result in a knockout mouse on a mixed background.