Abstract
Background
The most common ladybird beetle, Coccinella septempunctata L., is an excellent predator of crop pests such as aphids and white flies, and it shows a wide range of adaptability, a large appetite and a high reproductive ability. Diapause research plays an important role in the artificial propagation and shelf-life extension of insect products. Although this lady beetle’s regulatory, physiological and biochemical characteristics in the diapause period are well understood, the molecular mechanism of diapause remains unknown. Therefore, we collected female adults in three different states, i.e., non-diapause, diapause and diapause termination, for transcriptome sequencing.
Results
After transcriptome sequencing using the Illumina HiSeq 2500 platform with pretreatment, a total of 417.6 million clean reads from nine samples were filtered using the program FASTX (version 0.0). Additionally, 106,262 contigs were assembled into 82,820 unigenes with an average length of 921 bp and an N50 of 1,241 bp. All of the unigenes were annotated through BLASTX alignment against the Nr or UniProt database, and 37,872 unigenes were matched. We performed further analysis of these unigenes using the Clusters of Orthologous Groups of proteins (COG), Gene Ontology (GO), and the Kyoto Encyclopedia of Genes and Genomes (KEGG) databases. Through pairwise comparisons of the non-diapause (ND), diapause (D), and diapause-terminated (DT) groups, 3,501 and 1,427 differentially expressed genes (DEGs) were identified between D and ND and between DT and D, respectively. Moreover, 443 of the DEGs were specifically expressed during the diapause period (i.e., DEGs that were expressed at the highest or lowest levels during diapause compared with the other stages). GO function and KEGG pathway enrichment were performed on all DEGs and showed that RNA-directed DNA polymerase activity and fatty acid metabolism were significantly affected. Furthermore, eight specific expressed genes were selected for validation using qRT-PCR. Among these eight genes, seven genes were up-regulated, and one gene was down-regulated; the change trends of the eight genes were the same between the qRT-PCR and RNA-seq analysis results.
Conclusions
In this study, a new method for collecting and identifying diapause insects was described. We generated a vast quantity of transcriptome data from C. septempunctata L., providing a resource for gene function research. The diapause-associated genes that we identified establish a foundation for future studies on the molecular mechanisms of diapause.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-015-2309-3) contains supplementary material, which is available to authorized users.
Keywords: Coccinella septempunctata L, Diapause, Transcriptome, Fatty acid biosynthesis, Diapause-associated genes
Background
Both larval and adult Coccinella septempunctata L., the seven-spot ladybird beetle, are excellent predators of multiple aphid species. This species has been widely utilized as a biocontrol agent against various aphids because of its wide range of adaptability, large appetite, high reproductive ability and ease of mass rearing and has yielded numerous ecological and economic benefits [1–3]. The natural enemies of soybean aphids, such as lady beetles (Harmonia axyridis and C. septempunctata L.), lacewings (Chrysoperla carnea), and true bugs (Orius insidiosus), appear to play key roles in regulating aphid populations. Among these insects, C. septempunctata L. dominates early in the growing season [4–6]. In Europe, experiments on greenhouse crops, such as tomatoes, cucumbers and sweet peppers, have shown that C. septempunctata L. is one of the most important species of coccinellids in controlling aphid populations [2]. Pollution from chemical pesticides can be reduced by using lady beetles to control aphids. After late April or early May, the adults that have survived hibernation begin to spawn [7, 8].
Diapause is a genetic phenomenon in which an insect’s growth is almost terminated in response to one or more specific environmental stimuli and can only be reinitiated by another specific stimulus [9–11]; this is a behavior that allows insects to adapt to the environment. Once insects enter into the diapause state, they grow slowly, showing low metabolism or developmental stagnation for a long period in the absence of a specific stimulus to break diapause. Thus, the resistance of the insects is strengthened. Although these characteristics can make artificial propagation more challenging, they are also used to extend the shelf lives of insects. For example, in a study of the physiological characteristics of diapause in the lady beetle C. septempunctata L., the insect’s lifespan was significantly extended during diapause to more than 220 days, whereas the typical lifespan of this insect is approximately 63 days [12]. Numerous studies on diapause have elucidated the mechanisms of environmental regulation, particularly the role of the hormonal system in the occurrence and termination of diapause [13–15]. The diapause process can be divided into three phases: pre-diapause, diapause and post-diapause [16]. During the pre-diapause phase, insects perceive the triggering stimulus from the environment but continue to develop. Research on diapause induction in Bombyx mori has shown that rearing conditions of 25 °C with a 14-h light/10-h dark cycle can induce diapause [16, 17]. The external direct development of individual form (morphogenesis) begins to gradually slow and eventually ceases during diapause. Finally, during post-diapause, the insects immediately resume development when advantageous environmental conditions arise [18]. Many aspects of the physiological and ecological control of diapause are understood, but the molecular mechanism of diapause remains unclear [19, 20]. In recent decades, diapause research on various insects has been performed [21–23]. Some candidate genes have been found to play significant roles in the process of diapause. For example, a de novo transcriptome analysis of the Asian tiger mosquito, Aedes albopictus (Skuse), identified several genes, such as receptor for activated C kinase (rack1), ecdysone inducible protein L2 (eip) and G-protein coupled receptor (gpcr), that potentially play complex roles in diapause preparation [22]. In Culex pipiens, FOXO (forkhead box protein O) was found to play crucial roles in two characters during diapause: fat hypertrophy and extended lifespan [24]. There has been extensive research on B. mori diapause, and the diapause hormone was discovered and named in this species [25–27]. Analogs of this hormone have been found in Heliothis virescens and Helicoverpa armigera [28, 29], but their function contrasts with that of B. mori by ending diapause rather than inducing it. Among ladybugs, comprehensive research on diapause has primarily been conducted on adults of species such as Harmonia axyridis [30, 31] and Exochomus quadripustulatus. Some ladybugs show geographic diversity in diapause. Coccinella septempunctata L. exhibits summer diapause in Greece and winter diapause in central Western Europe [32, 33]. Studies on C. septempunctata L. in Beijing have revealed the occurrence of winter diapause, with the highest proportion of diapause occurring under rearing conditions of 18 ± 1 °C with a 10:14 (L:D) h photoperiod [30]. The physiological and ecological controls of diapause are known [6, 34], and the differential expressions of diapause-related proteins in C. septempunctata L. have been studied in our laboratory [35], whereas the molecular bases of diapause remain unclear. Recently, next-generation sequencing technology has greatly promoted the development of insect transcriptomics, especially for insects without reference genome sequences [36–38].
Next-generation sequencing technology (NGS), also known as high-throughput sequencing technology, collects massive amounts of sequence data in a single run [39, 40]. The emergence of NGS has greatly promoted the study of insect transcriptomes. To date, the transcriptomes of 68 species from 7 insect orders have been sequenced [41–43], and the transcriptome and expression profile data of some lady beetles such as Cryptolaemus montrouzieri Mulsant and Propylaea japonica (Thunberg) have been analyzed [44, 45]. For organisms without reference genomes, de novo transcriptome sequencing can not only produce genetic information for the species but also be used to predict possible non-coding RNA [46, 47]. Transcriptome sequencing of insect samples obtained under different growing conditions or from different habitats or species not only generates functional gene information and reveals differentially expressed genes but also provides the abundance of expressed transcripts, the loci where transcription occurs, transcript SNPs and other important information [48–51]. We conducted research on diapause in C. septempunctata L. based on the results of other insect studies [52].
In this study, we used RNA sequencing to identify differentially expressed genes related to diapause by comparing non-diapause, diapause, and diapause-terminated groups; a total of 461 diapause-associated genes (DGs) were identified (i.e., DEGs that were expressed at the highest or lowest levels during diapause compared with the other stages). To verify the precision of the transcriptome results, we selected 8 DGs via quantitative real-time PCR (qRT-PCR): sorbitol dehydrogenase (sdh), glycogen phosphorylase (gp), toll-like receptor 2 (tlr2), serine protease (sp), juvenile hormone epoxide hydrolase (jheh), acetyl-CoA carboxylase (acc), trehalase (tre) and an uncharacterized protein, LOC100142139. These DGs were specifically expressed in diapause and play important roles in energetic metabolism, immune defense, hormone regulation, and other functions. We discuss the molecular functions and regulatory mechanism of the DGs relative to those from other insects in this manuscript. Some significant pathways, such as the insulin/FOXO signaling pathway and the cell cycle, were also analyzed. The transcriptome results for C. septempunctata L. enrich the available information on insect diapause and provide an improved method for collecting and identifying insects in diapause.
Results and discussion
Results
Experimental insects in diapause
In adult insects, the most obvious and easily recorded feature of diapause is that reproduction is inhibited. Current research indicates that adult diapause can be reflected by specific indices, such as the time of pre-spawning, the developmental process of the ovary and ova, reproductive activity, and body surface features [53, 54]. In Hokkaido, Japan, it was found that the ovarian development of adult C. septempunctata L. females nearly ceases and that the eggs in the ovary are immature. Moreover, egg cell development is inhibited, and the developmental stages usually do not exceed the period of yolk formation [55]. A study from our laboratory examining the preliminary ecology of C. septempunctata L. in Beijing, China, showed that diapause occurred in adult C. septempunctata L. We further found that the adult diapause rate was highest when the temperature and photoperiod conditions were 18 °C and 10:14 h (L:D), respectively, and that the female ovary was transparent, ovarioles showing yolk deposition were absent, and the egg tubules were not obviously differentiated under these conditions [56]. Therefore, in the present study, all of the experimental insects in diapause were reared under a normal developmental temperature of 24 °C, a photoperiod of 16:8 h (L: D) and an RH of 70 ± 10 % until emergence (Fig. 1). After emergence, the insects were immediately transferred to another artificial climate box with a temperature and photoperiod of 18 °C and 10:14 h (L: D), respectively, and 2 female and 2 male insects were bred in one plastic cup for 30 days. Then, one insect was randomly selected for dissection to observe ovary development; the other female adult insect was identified as a diapausing individual because the ovary was undeveloped.
Fig. 1.
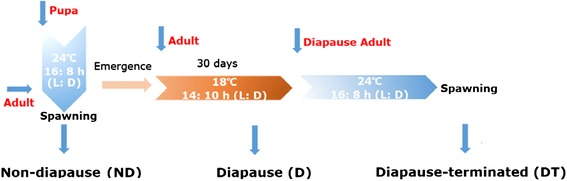
Schema of sampling for transcriptome sequencing. All the insects are reared at 24 °C with long-day from egg to pupa and emergence for sampling
Illumina sequencing and de novo assembly
According to the data obtained through sequencing using the Illumina HiSeq 2500 platform, we performed routine transcriptome analysis on adult female C. septempunctata L. in different developmental stages: non-diapause, diapause, and diapause-terminated. With three biological replicates of three samples, the 9 libraries yielded 453,401,612 raw reads, comprising 154,790,202, 145,249,536 and 153,361,874 reads for the non-diapause (ND), diapause (D) and diapause-terminated (DT) groups, respectively (Table 1). After adaptor trimming and quality filtering, a total of 417,628,298 clean reads were filtered, and 106,262 contigs were assembled using CLC Genomics Workbench (version 6.0.4), with a mean length of 713 bp (Table 1). After the assembly of one or more contigs, 82,820 unigenes were generated with an average length of 921 bp (Additional file 1). The lengths of the assembled unigenes were primarily in the range of 200–1,000 bp (approximately 72.67 % of the total unigenes were in this range), with most unigenes falling between 401 bp and 600 bp and some exceeding 2000 bp (Fig. 2). The results of open reading frame (ORF) prediction demonstrated that 38,488 of 82,820 unigenes contained one or more ORFs.
Table 1.
Summary statistics from Illumina sequencing of the C. septempunctata L. transcriptome
| Sequencing | |
|---|---|
| Total number of reads | 453,401,612 |
| Number of clean reads | 417,628,298 |
| Number of contigs | 106,262 |
| Total length of contigs (bp) | 75,724,113 |
| Contig N50 | 929 |
| Average length of contigs (bp) | 713 |
| Number of primary unigenes | 84,291 |
| Number of final unigenes | 82,820 |
| Total length of final unigenes (bp) | 76,279,917 |
| Final unigene N50 | 1,241 |
| Average length of final unigene (bp) | 921 |
| GC percentage (%) | 42. 4 |
| N percentage (%) | 1. 2 |
| Annotation | |
| Unigene annotations against Nr | 35,115 |
| Unigene annotations against UniProt | 37,415 |
| Total number of Unigene annotations | 37,872 |
| Functional classifying and pathway mapping | |
| Unigene annotations against KEGG | 18,286 |
| Unigene annotations against GO | 112,402 |
| Unigene annotations against COG | 100,308 |
Fig. 2.
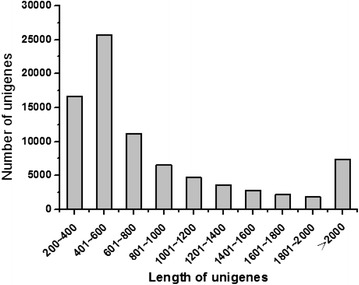
Length distribution of unigenes. A total of 82,820 unigenes were assembled. The x-axis represents the length, and the y-axis represents the number of unigenes
Annotation of unigenes
When the unigenes were annotated according to the UniProt and Nr databases using BLASTX, 37,872 of 82,820 unigenes were annotated (approximately 45.70 % of all of the unigenes), and 37,415 contigs were filtered with a cutoff e-value of 1e-5, among which 46.2 % of the homologous sequences showed values below 1e-50 (Fig. 3a). Because the genome sequence of C. septempunctata L. has not been reported, sequence alignment of the experimental unigenes was performed using the known genomes of other species. In the species distribution, the top matches were obtained for Tribolium castaneum, with 10,655 sequences (28.5 %), followed by Acyrthosiphon pisum (9.03 %), Anoplophora glabripennis (7.07 %), Dendroctonus ponderosae (6.71 %), Acanthamoeba castellanii str. Neff. (3.01 %) and other species (45.68 %) (Fig. 3b).
Fig. 3.
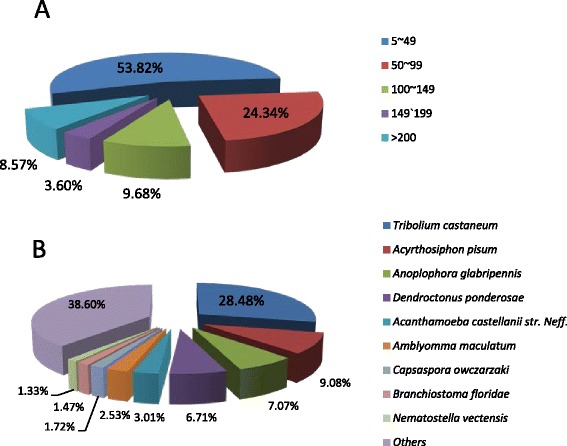
Homology searches of Illumina sequences against the UniProt and Nr database. a E-value distribution. The filtering conditions for the unigenes matched against the UniProt and the Nr databases were set to a cut-off value of 1e-5. The range of 5–10 represents 1e-5-1e-10. b Species distribution of BLASTX hits. The species were determined based on the highest score in the Bx results
To further analyze the integrity of the libraries and the effectiveness of the annotation process, COG functional classification was performed on the unigene alignment with the CDD database [57] using the rpstBlastn program; 32,350 unigenes were annotated to 25 COG categories (Fig. 4). The largest group in the cluster was “signal transduction mechanisms”, with 19,785 unigenes; followed by “RNA processing and modification”, with 18,209 unigenes; “cytoskeleton”, with 16,201 unigenes; “general function prediction only”, with 12,382 unigenes; and “posttranslational modification, protein turnover, chaperones”, with 7,688 unigenes. These results demonstrated that signaling transduction mechanisms were the most important in adult female C. septempunctata L.. Gene Ontology (GO) is an international standardization of the gene functional classification system. Improvement of the biological annotation of genes contributed to the development of GO classification, and the three-class system (biological processes, molecular functions, and cellular components) plays a key role in the bioinformatics annotation process. In this experiment, biological processes, molecular functions, and cellular components were associated with 20,990 unigenes, 22,866 unigenes, and 9,961 unigenes, respectively (Additional file 2). Among biological processes, metabolic processes (29.26 %) and cellular processes (24.38 %) were the most abundant groups, whereas immune system processes (0.02 %), growth (0.05 %) and locomotion (0.06 %) were the least abundant groups (Fig. 5a). In terms of molecular functions, binding (45.29 %) and catalytic activity (40.91 %) accounted for the majority of unigenes in the unigene classification, whereas protein tags (near 0.00 %), metallochaperone activity (0.01 %), and nutrient reservoir activity (0.02 %) were the least abundant categories (Fig. 5b). Among the cellular components, the cell (20.78 %) and cell part (20.78 %) categories were the most abundant, followed by the organelle (14.82 %) and membrane (14.49 %) categories, whereas extracellular matrix (0.03 %) and collagen (0.07 %) were the least abundant categories (Fig. 5c).
Fig. 4.
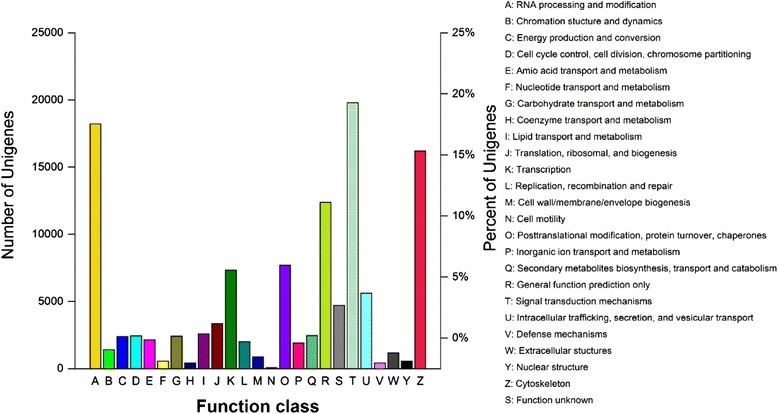
Clusters of Orthologous Groups (COG) functional categories in the C. septempunctata L. transcriptome. A total of 32,350 unigenes were annotated into 25 categories. The left y-axis represented number of unigenes, and right y-axis represented percent of unigenes
Fig. 5.
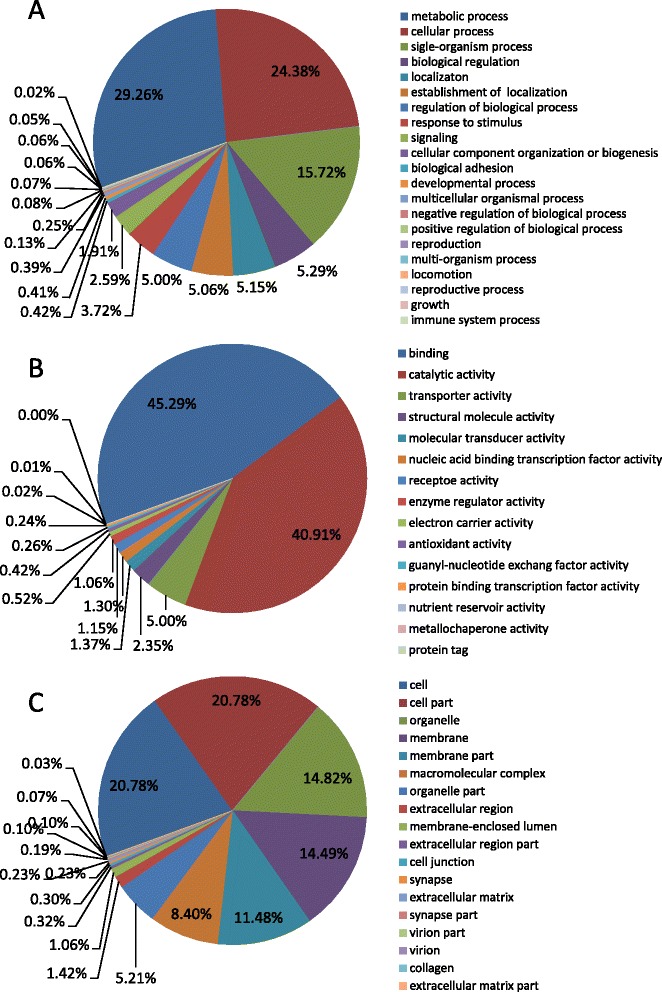
Gene ontology (GO) classifications for the C. septempunctata L. transcriptome. In the graph, a, b, and c represent biological processes, molecular functions, and cellular components, respectively
When the biologically complex activity of genes was examined using KEGG, 11,949 unigenes were mapped to 308 pathways based on matching with the KAAS (KEGG Automatic Annotation Server). Among these pathways, metabolic pathways, biosynthesis of secondary metabolites, and microbial metabolism in diverse environments were the most represented, with 3,800 unigenes, 1,600 unigenes, 1,217 unigenes, respectively. We identified the areas of interest to further analyze these annotations, providing a valuable resource for elucidating functional genes in adult female C. septempunctata L..
Analysis of gene expression profiles
To identify significant expression changes in genes, we conducted a differential expression analysis of unigene expression through pairwise comparisons of the three different conditions, non-diapause, diapause, and diapause terminated, and the DEGs were filtered using an FDR (false discovery rate) ≤0.05 and a fold change ≥2. Because of the slow pace at which physiological activities and growth occur during the diapause stage, most genes were silent; however, the expression of some genes remained up-regulated. When ND and D were compared, 2,125 up- and 1,376 down-regulated unigenes were revealed, and 749 up- and 678 down-regulated unigenes were detected between D and DT. To filter the DEGs related to diapause, we examined the unigenes showing higher or lower expression levels in D compared with ND and DT. A total of 443 up- and 18 down-regulated unigenes in the diapause phase were identified based on the annotation results (Fig. 6). Additionally, the up-regulated DEGs were classified into five categories with 27 terms via KEGG Orthology (KO) (Fig. 7).
Fig. 6.
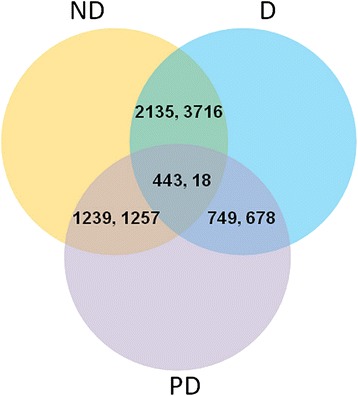
Summary of differentially expressed genes in three libraries identified through pairwise comparisons. The overlapping areas represented the genes we filtered by comparing two or three groups. In each pair of values, the first pair represents the number of up-regulated unigenes, and the second pair represents the number of down-regulated unigenes. ND: non-diapause, D: diapause, DT: diapause-terminated
Fig. 7.
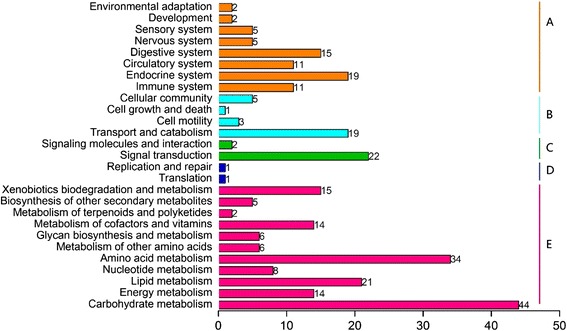
KEGG Orthology (KO) classifications of up-regulated differentially expressed genes (DEGs). 443 DEGs were mapped to five categories; a: Organismal Systems; b: Cellular Processes; c: Environmental Information Processing; d: Genetic Information Processing; e: Metabolism
Functional enrichment analysis of differentially expressed genes
To understand the functions of the differentially expressed genes, we compared the GO terms associated with the three different treatments after mapping all of the differentially expressed genes to the GO database. In D/ND and DT/D, 793 GO terms (including 289 biological processes, 421 molecular functions, and 83 cellular components) and 459 GO terms (including 173 biological processes, 240 molecular functions, 46 cellular components), respectively, classified all of the differentially expressed genes. We selected 186 terms as significantly enriched terms for D/ND and 155 such terms for DT/D to further comprehend the function of the differentially expressed genes with a cutoff FDR of 0.05. Moreover, we performed an analysis of significantly enriched pathways using a hypergeometric test, comparing the whole genomic background to detect the significantly enriched pathways of the differentially expressed genes. The results showed that 1,174 differentially expressed unigenes and 350 differentially expressed unigenes mapped with 261 and 233 pathways on D/ND and DT/D, respectively. Among these, 74 pathways and 19 pathways were significantly enriched with the threshold set to an FDR ≤0.05 (Table 2 and Additional file 3) (the threshold for D/ND was decreased to FDR ≤0.001 due to the excess number of significantly enriched pathways; the remaining pathways are listed in Additional file 3). A comprehensive comparative analysis of these significantly enriched pathways showed that fatty acid metabolism was the most significantly enriched pathway.
Table 2.
significantly enriched KEGG pathways in D vs. ND and DT vs. D
| Comparison | KEGG pathway | Up genes | Down genes |
|---|---|---|---|
| D vs ND | Fatty acid metabolism | 55 | 8 |
| Biosynthesis of secondary metabolites | 174 | 74 | |
| Citrate cycle (TCA cycle) | 41 | 7 | |
| Carbon metabolism | 78 | 27 | |
| Pyruvate metabolism | 40 | 13 | |
| Ribosome biogenesis in eukaryotes | 0 | 2 | |
| Glycolysis / Gluconeogenesis | 44 | 18 | |
| Fatty acid biosynthesis | 33 | 5 | |
| Galactose metabolism | 27 | 8 | |
| Cardiac muscle contraction | 36 | 0 | |
| Biosynthesis of amino acids | 60 | 28 | |
| Starch and sucrose metabolism | 41 | 22 | |
| Oxidative phosphorylation | 64 | 8 | |
| Lysosome | 48 | 9 | |
| Carbon fixation in photosynthetic organisms | 24 | 18 | |
| beta-Alanine metabolism | 22 | 8 | |
| Ribosome | 127 | 36 | |
| Carbon fixation pathways in prokaryotes | 22 | 4 | |
| Propanoate metabolism | 23 | 0 | |
| Fatty acid degradation | 28 | 5 | |
| Fructose and mannose metabolism | 32 | 13 | |
| Signaling pathways regulating pluripotency of stem cells | 0 | 2 | |
| One carbon pool by folate | 26 | 2 | |
| Drug metabolism - other enzymes | 26 | 2 | |
| Spliceosome | 4 | 14 | |
| Meiosis - yeast | 1 | 1 | |
| Cell cycle | 0 | 7 | |
| Phagosome | 34 | 17 | |
| Aminoacyl-tRNA biosynthesis | 8 | 4 | |
| Aflatoxin biosynthesis | 10 | 0 | |
| Nucleotide excision repair | 0 | 1 | |
| Ras signaling pathway | 3 | 1 | |
| AMPK signaling pathway | 40 | 10 | |
| Epstein-Barr virus infection | 4 | 12 | |
| Arginine and proline metabolism | 25 | 9 | |
| TD vs D | Cardiac muscle contraction | 0 | 14 |
| Drug metabolism - other enzymes | 2 | 11 | |
| Fatty acid metabolism | 2 | 18 | |
| Carbon fixation pathways in prokaryotes | 0 | 11 | |
| Spliceosome | 1 | 0 | |
| Ribosome | 3 | 9 | |
| MicroRNAs in cancer | 12 | 6 | |
| Citrate cycle (TCA cycle) | 1 | 14 | |
| Styrene degradation | 0 | 4 | |
| Vitamin digestion and absorption | 2 | 3 | |
| Tryptophan metabolism | 1 | 9 | |
| Tyrosine metabolism | 1 | 8 | |
| Cutin, suberine and wax biosynthesis | 1 | 5 | |
| One carbon pool by folate | 1 | 9 | |
| Photosynthesis - antenna proteins | 0 | 9 | |
| Fatty acid degradation | 1 | 10 | |
| Insulin signaling pathway | 8 | 10 | |
| Novobiocin biosynthesis | 0 | 2 | |
| Adrenergic signaling in cardiomyocytes | 0 | 13 |
Note: In D vs. ND, all FDR ≤ 0.001; and in TD vs. D, all FDR ≤ 0.05
Fatty acid biosynthesis
Adults of C. septempunctata L. feed during diapause; however, their food intake is 80 % lower than that of non-diapause adults [36]. Therefore, the accumulation of nutrients is crucial before entry into diapause. Lipids store metabolic reserves of macronutrients, and triacylglycerides are the primary form of storage lipids in insects [58]. One remarkable physiological characteristic of C. septempunctata L. in diapause is fat hypertrophy. Furthermore, fatty acids are one of the substrates of triacylglyceride synthesis. In comparisons of the KEGG enrichment pathways identified between D/ND and DT/D, we found that there were many more up-regulated genes than down-regulated genes. Among these pathways, all of the 9 DEGs in the KEGG enrichment pathways identified between DT/D were down-regulated; therefore, we filtered the 9 unigenes (7 fatty acid synthase and 2 long-chain acyl-CoA synthetases) with a fold change ≥2 and an FDR ≤0.05, which were up-regulated at diapause and down-regulated at diapause termination (Additional file 4).
Validation of unigenes using qPCR
The 8 DEGs sorbitol dehydrogenase (sdh), glycogen phosphorylase (gp), toll-like receptor 2 (tlr2), serine protease (sp), juvenile hormone epoxide hydrolase (jheh), acetyl-CoA carboxylase (acc), trehalase (tre) and the uncharacterized protein LOC100142139 were validated through quantitative real-time PCR (Additional file 5). The results of all gene validations are shown in Fig. 8. In Fig. 8a, the RPKM values for RNA-seq and the fold changes detected through qRT-PCR are shown in a single chart to facilitate comparison of the differences. Additionally, the change trends of the 8 DEGs at 6 diapause periods (D1, D2, D3, D4, D5 and D6) are shown in Fig. 8b. Peak expression was observed during diapause at 30 or 40 days except for SP. In general, the results of qRT-PCR verified the reliability of the de novo transcriptome sequencing of C. septempunctata L. as the validation results were consistent with transcriptome sequencing regarding the expression level at the three stages.
Fig. 8.
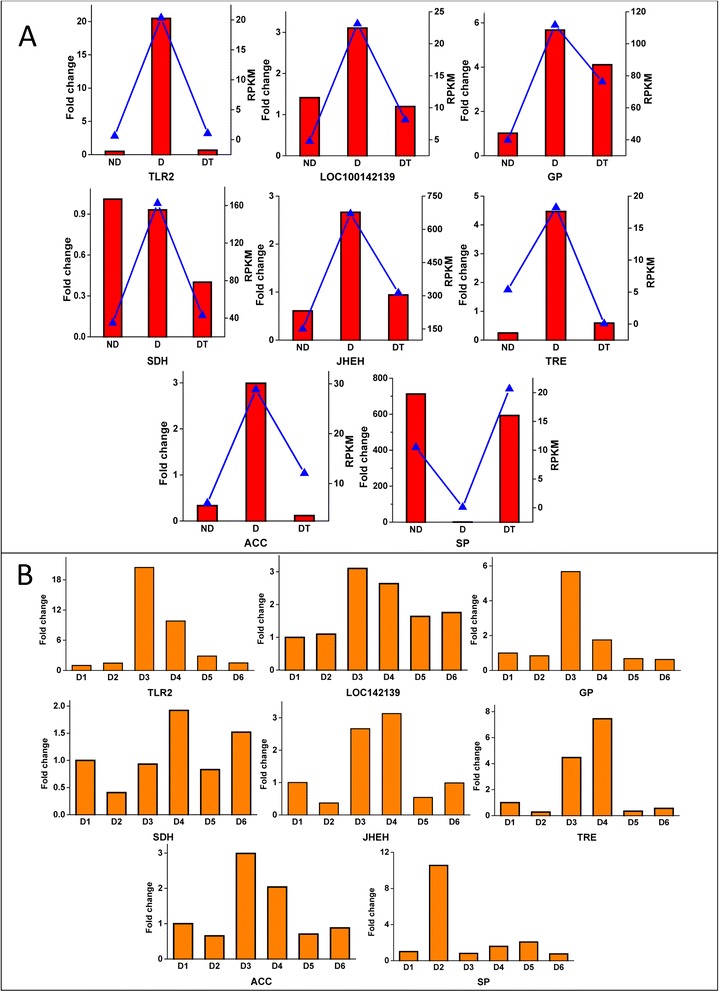
Validation of candidate differentially expressed genes. a Expression of differentially expressed genes compared between the qRT-PCR and the RNA-seq results in the three stages: non-diapause, diapause and diapause-terminated. The qRT-PCR results are shown in red, and the values are shown on the left y-axis; the RNA-seq results are shown with blue lines, and the values correspond to the right y-axis. b qRT-PCR validation of 6 treatments at diapause stages D1, D2, D3, D4, D5, and D6,correspondingto 10, 20, 30, 40, 50, and 60 days, respectively
Discussion
Insect diapause may be triggered by various hostile environmental conditions, sudden changes in temperature or photoperiod, or shortage of food [11, 19]. Studies of the regulation of diapause have demonstrated that a number of genes are silent or uniquely expressed during the diapause period; for example, in Sarcophaga crassipalpis, hsp70 (heat shock protein) is uniquely expressed while hsp90 is lowly expressed at the diapause stage [59, 60]. These genes have been reported to be involved in the change of biochemical pathways, changes in metabolic products, energy production, and stress-tolerance enhancement. Usually, diapause is restricted to a specific stage, such as the larva or adult stage [19]. Although the characteristics and functions of a few diapause-associated genes vary with diapause stage, our study on the regulation diapause-associated genes in C. septempunctata L. might be useful for future studies of the molecular mechanisms in other species. However, because of the lack of information on gene functions and other characteristics, approximately 54.3 % unigenes were not annotated to any functional genes in our study; their functions will be addressed in our subsequent studies. The present study had some limitations. Although a low temperature is the key condition for inducing artificial diapause in ladybirds, differences in temperature among the diapause groups might have induced variation in developmental kinetics, which subsequently might have induced variation in gene expression. In addition, diapause-terminated females are 10 to 20 days older than are diapausing females because of the time required to break diapause and spawn following the presentation of specific external environmental stimuli: 10 to 20 days are required following the changes in temperature and photoperiod. Diapause is a process in which the stages of diapause, i.e., pre-diapause, diapause, and post-diapause, and the breaking of diapause occur chronologically; therefore, the studied diapause-terminated females were older than the diapausing females. The potential difference in gene expression due to this age difference cannot be eliminated from our experiment. To investigate whether the filtered genes are diapause-associated genes or are genes associated with other, age-related processes, more advanced biological techniques for functional analysis are needed.
Diapause-associated genes identified by comparing among the three stages
We filtered 461 DEGs by comparing the D group to the ND and DT groups. Among these DEGs, 443 were up-regulated in diapause and were mostly involved in carbohydrate metabolism, amino acid metabolism, signal transduction, and lipid metabolism; carbohydrate metabolism was most commonly represented, with 44 DEGs involved (Fig. 7). Many genes were involved in different pathways. Seven diapause-associated genes with high differentially expression values were tlr2, jhe, jheh, sdh, tre, fah and sp. With the exception of sp, all of these genes were up-regulated during diapause. Some of these genes have been not reported previously in insect diapause. For example, Toll-like receptors (TLRs) are key components of innate immunity and the activation of adaptive immunity [61]. Contig 19695 was predicted to encode TLR2, and the expression level in D was up-regulated by more than 32-fold and 19-fold compared with ND and DT, respectively. TLR2 is a membrane protein involved in several signal transduction pathways, such as the Toll-like receptor signaling pathway, and plays an important role in the immune surveillance of various organs in mammals and fish [62]. Fumarylacetoacetase (FAH, Contig 14637 and Additional file 5) is the final and rate-limiting enzyme in tyrosine degradation. Tyrosine is a substrate for melanogenesis through tyrosine metabolism. In melanin cells, the conversion of tyrosine into dopa are catalyzed by tyrosinase, after which oxidation generates dopaquinone to enter the pathway of melanogenesis [63]. Further research on the function of FAH in insect diapause is needed.
Studies of diapause in other insects suggest that it is regulated and controlled by hormones [19]. jheh (Contig 105) and juvenile hormone esterase (jhe, Contig 7594 and Additional file 5), the enzymes responsible for juvenile hormone (JH) metabolism, regulate the titer of JH in the hemolymph. Based on the results of the transcriptome analysis, we found that the expression value of jheh increased during diapause and decreased after diapause. Most studies of JH and diapause show that the absence of JH is the main factor controlling diapause without the corpora cardiaca-corpora allata (CC-CA) complex in insects [64]. Furthermore, the levels of JH III in the hemolymph of pre-diapause and diapause adults were revealed to be low compared to normal developmental adults under long-day photoperiod using JH titer determinations, and the activity correlation between JHE and JH was found to be negative in the Colorado potato beetle, Leptinotarsa decemlineata [65–67]. The up-regulation of JHEH and JHE may be due to the decline in JH in vivo during diapause in the adult blow fly Protophormia terraenovae [68]. We found that sdh (Contig 2214) expression in the D group was 4 times higher than that in ND and DT. In Wyeomyia smithii, the expression of SDH during larval diapause is high, and after diapause, its expression is reduced. Conversely, SDH is expressed at low levels in the silkworm B. mori during egg diapause and has been shown to play a key role in diapause termination [69]. These results indicate that changes in SDH expression are not consistent among different diapause types. TRE (Contig16915), a glycoside hydrolase enzyme, catalyzes the conversion of trehalose into glucose. In C. septempunctata L., it is expressed more during diapause than in the two other phases. Many reports have indicated that in B. mori, trehalase activity in the ovaries is controlled by a diapause hormone during the diapause stage and that this hormone enhances the transcriptional activity of the trehalase gene in developing ovaries [70, 71]. According to the results of the DEGs, the number of genes that were down-regulated during diapause was significantly lower than the number of up-regulated genes in C. septempunctata L.. SP (Contig 1935), an important hydrolytic and digestive enzyme in insects, was found to be down-regulated during diapause in C. septempunctata L., with the RPKM value during diapause being 100 times lower than that during the non-diapause stage and 500 times lower than that in the diapause-terminated stage. Trypsin and chymotrypsin-like serine protease, genes encoding two blood-digestive enzymes in the class of serine proteases, were down-regulated in diapause-destined females in the mosquito C. pipiens, suggesting that the expression of genes associated with the digestion of a blood meal has been shut down [72].
Diapause-associated pathways
In B. mori and S. crassipalpis, the cell cycle is arrested during diapause [73]. Moreover, studies on cell cycle regulatory genes in the brain of S. crassipalpis during diapause have shown that cell nuclear antigen (PCNA) is expressed at very low levels. In contrast, during the non-diapause or diapause-terminated stage, the expression of PCNA is high. Based on KEGG enrichment analysis of D vs. ND and DT vs. D, we showed that all seven DEGs were down-regulated in D compared with ND and that no DEGs were up-regulated in D. Furthermore, a total of 15 DEGs were annotated in the comparison of DT and D, including wee1-like protein kinase [EC:2.7.11.1], polo-like kinase 1 [EC:2.7.11.21], DNA replication licensing factor MCM6 [EC:3.6.4.12], cyclin A/B (CYCA/B), S-phase kinase-associated protein 1/2 (SKP1/2), cell division cycle 20 (CDY20), anaphase-promoting complex subunit 1 (APC1), and origin recognition complex subunit 5 (ORC5), all of which were up-regulated in DT (Additional file 4). Thus, cell cycle arrest might be a hallmark of ladybug diapause, although the specific phase was undefined in C. septempunctata L. The insulin signaling pathway has been the subject of much research in insect diapause. Studies on insulin signaling might be a unifying direction for diapause research. Work examining diapause in Drosophila melanogaster and the mosquito C. pipiens revealed that the insulin signaling pathway is involved in the regulation of the diapause phenotype. More recent studies have discussed the regulatory phenotype of diapause, including lifespan extension, stagnated reproduction, suppressed metabolism, and enhanced stress tolerance and fat accumulation. FOXO, a forkhead transcription regulatory factor, plays a key role in growth and development, metabolism, aging and immunity. For example, in Drosophila, activated dFOXO is involved in G1 cell cycle arrest [74]. In C. pipiens, research on the regulation of insulin signaling and FOXO indicates that under normal developmental conditions, insulin signaling prompts the synthesis of JH, thus stimulating the development of the ovaries. Concurrently, FOXO expression is restrained, leading to the suppression of fat accumulation. Conversely, during diapause, insulin signaling is suppressed, and the JH titer is down-regulated, leading to up-regulation of FOXO expression, promoting fat hypertrophy. Our results indicate that Contig 13394 is annotated as forkhead box protein O3 (FOXO3), which is up-regulated during diapause compared with non-diapause and diapause-terminated stages (Additional file 1), whereas the FDR of Contig 13394 was not within the limited range (≤0.05). Therefore, the specific regulation of insulin signaling and FOXO in C. septempunctata L. during diapause must be determined through additional methods, such as RNA interference (RNAi), which will be the emphasis of future work on the molecular mechanisms of ladybug diapause.
Conclusions
In this study, we presented a simple method for collecting and identifying experimental insects in diapause. In addition, we performed Illumina sequencing of C. septempunctata L. in different developmental stages, followed by read cleaning, de novo assembly and functional annotation, which yielded 82,820 unigenes, among which 37,872 were annotated. The most closely matched species was T. castaneum (10,655 unigenes), which suggests a close relationship between C. septempunctata L. and T. castaneum. Furthermore, we revealed that the type and expressed quantity of genes varied among the three different developmental stages based on pairwise comparisons. A total of 443 genes of interest were identified that had higher expression levels during diapause than during the other developmental stages. Additionally, many of these candidate genes were found to be involved in metabolism, signal transduction and hormonal control of development. Finally, we validated the transcriptome results via qRT-PCR for eight specific expressed genes. The change trends of these eight genes as identified through qRT-PCR were consistent with the RNA-seq analysis results, verifying the robustness of our results. These results increase our knowledge of the molecular biology of ladybug diapause and enhance our understanding of the molecular mechanisms of diapause.
Methods
Insects
Coccinella septempunctata L. were captured in the wheat fields of the Institute of Crop Sciences of the Chinese Academy of Agricultural Sciences and cultured in a climatic cabinet. The experimental insects were reared in plastic containers in the laboratory and fed soybean aphids daily. The insects undergo imaginal diapause; therefore, in the egg, larva and pupa stages, the rearing conditions were a temperature of 24 ± 1 °C, an RH of 70 ± 10 % and a 16:8-h light:dark (L:D) photoperiod [8, 34, 56]. Non-diapause female insects were also reared under these conditions and collected after their first oviposition. Female adults in diapause were reared at 18 ± 1 °C at an RH of 70 ± 10 % and a 10:14-h (L:D) photoperiod [56]. The experimental diapause insects were collected after 30 days. Diapause-terminated adults were transferred to another climatic cabinet with the 30-day diapause insects and reared under the same conditions as the non-diapause insects. After their first oviposition, the female insects were collected and stored at −80 °C. A Chinese patent is pending for the methods for diapause regulation and the storage and maintenance of C. septempunctata L. [75, 76].
RNA preparation, cDNA library construction and RNA sequencing
Total RNA was extracted using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions, and the RIN was assessed to inspect RNA integrity using an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). The qualified total RNA was further purified with an RNeasy micro kit (Qiagen, Valencia, CA, USA) and an RNase-Free DNase Set (Qiagen, Valencia, CA, USA).
After the extraction of total RNA, oligo (dT) magnetic beads were used to isolate poly (A) mRNA, which can break mRNA into short fragments when fragmentation buffer is added. Employing these short fragments as a template, first-strand cDNA synthesis was performed using random hexamer primers, after which second-strand cDNA was synthesized in reactions including buffer, dNTPs, RNase H and DNA polymerase I. End repair of the adenylated 3’ ends was conducted on the cDNA fragments, and adapters were ligated following purification with a QIAquick PCR purification kit (Qiagen, Hilden, Germany); the fragments were then eluted with EB buffer. Suitable fragments were selected via agarose gel electrophoresis and amplified through PCR to generate the cDNA library. Finally, the concentration and size of the cDNA library was determined with a Qubit 2.0 Fluorometer (Invitrogen, Carlsbad, CA, US) and an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). After quality inspection, sequencing was performed with the Illumina HiSeq 2500 system (Illumina, San Diego, CA, USA). Three biological replicates per treatment (non-diapause, diapause, diapause-terminated) were sequenced. The base period and mass fraction resulting from high–throughput sequencing were recorded in FASTQ format. The results of the quality evaluation of the sequencing data were excellent, with bases being distributed evenly; thus, we continued our analyses. Sequencing of the different developmental stages of ladybird adults produced raw reads.
De novo transcriptome assembly and functional annotation
Raw reads were transformed from the original sequencing image data through base calling. The clean reads were filtered to remove reads with adaptors, low-quality reads (ratio of greater than 20 bases, less than 50 %), and reads showing an N ratio (unknown sequences) greater than 5 % using the FastX program (version 0.0.13) [77]. A pool of reads was formed by merging nine samples of sequencing data. De novo transcriptome assembly of the clean reads was performed using the scaffolding contig algorithm of CLC Genomics Workbench (version: 6.0.4) [78]. The parameters of the scaffolding contig algorithm were as follows: Word-size = 45 and minimum contig length ≥300. The final unigenes were synthesized through a second assembly, which was performed with the assembly program CAP3 EST. The final unigenes were then annotated by searching against the UniProt or Nr database using the BLASTX program [79]. We performed annotation through COG (Cluster of Orthologous Group of proteins), GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) mapping. The GO and KEGG classifications were performed using BLASTX and KAAS [80]. The expectation value (E-value) was less than 1 × 10−5. The COG functional categories were adopted from the rpstBlastn program based on comparison with unigenes in the CCD database. Blast2GO [81] was employed for GO functional categories according to the Gene Ontology terms molecular function, cellular component and biological process.
Differential gene expression profiling of non-diapause, diapause and post-diapause stages
For the differential gene expression analysis, we obtained the genes with differentially expressed counts in different samples and performed GO functional analysis and KEGG pathway analysis of the differentially expressed (DE) genes. The RPKM (reads per kb per million reads) method [82] was used to calculate the expression counts of the unigenes. This method can eliminate the influence of expression levels due to differences in the lengths and amounts of sequences. The multiples of differential expression levels between different samples for a gene were calculated using the RPKM method. In addition, the RPKM of each sample as an internal standard was subjected to the Fisher test using the R language. Differentially expressed genes were identified using the Fisher test with FDR correction of the significance values. The DEGs were filtered with the following requirements: FDR ≤0.05,fold change ≥2. For pathway enrichment analysis, all of the differentially expressed genes were mapped to GO and KEGG pathway terms and the significantly enriched terms were filtered. For DEGs, GO enrichment analysis can determine the key biological functions and KEGG can determine the key biochemical metabolic pathways and signal transduction pathways.
Quantitative real-time PCR (qRT-PCR) validation
Seven up-regulated genes and 1 down-regulated gene that were expressed during diapause were validated and quantified via real-time PCR, with three biological replicates and three technical replications. The total RNA was extracted with TRIzol reagent (Life Technologies, Carlsbad, CA, USA) for the different developmental stages (non-diapause, diapause and diapause-terminated). The diapause stage was divided into 6 phases, D1, D2, D3, D4, D5 and D6, representing diapause after 10, 20, 30, 40, 50, and 60 days, respectively. Then, 1 μg of RNA was employed for first-strand cDNA synthesis using the Prime ScriptII 1st Strand cDNA Synthesis Kit (Takara, Dalian, China) according to the manufacturer’s protocol. Real-time PCR was conducted using SYBR FAST qPCR Kit Master Mix (2×) Universal (KAPA Biosystems, Woburn, MA, USA) under the following conditions: 95 °C for 5 min, followed by 40 cycles of 95 °C for 30 s and 60 °C for 20 s. The melting curve was analyzed from 60 °C to 95 °C to detect nonspecific product amplification. The 18S-RNA gene of C. septempunctata L. was used as an internal gene. The primers used for real-time PCR are listed in Additional file 6. All of the data obtained through qRT-PCR were analyzed via the 2-ΔΔCT method.
Availability of supporting data
All Illumina data used in the present study have been deposited in NCBI’s Gene Expression Omnibus (GEO) under accession number GSE75645.
Acknowledgements
We would like to thank Bohao (Shanghai Biotechnology Corporation) for its assistance of origin data processing and related bioinformatics analysis. We also thank Sha Jiang, Mengqin Wang, Chenxi Liu, and members of Biocontrol lab for suggestion and discussion of this work and manuscript revision. This research was supported by the National Natural Science Foundation of China (31572062), the National Basic Research Program of China (973 Program, 2013CB127602), and Special Fund for Agro-scientific Research in the Public Interest (201103002).
Abbreviations
- BLAST
Basic local alignment search tool
- COG
Clusters of orthologous groups of proteins
- GO
Gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- ND
Non-diapause
- D
Diapause
- DT
Diapause-terminated
- DEGs
Differentially expressed genes
- qRT-PCR
Quantitative real-time polymerase chain reaction
- RNA-Seq
RNA sequencing
- FOXO
Forkhead box protein o
- L: D
Light: dark
- NGS
Next-generation sequencing technology
- DG
Diapause-associated gene
- ORF
Open reading frame
- CDD
Conserved domain database
- KAAS
KEGG automatic annotation server
- FDR
False discovery rate
- RPKM
Reads per kb per million reads
- TLR
Toll-like receptor
- FAH
Fumarylacetoacetase
- JH
Juvenile hormone
- JHE
Juvenile hormone esterase
- SDH
Sorbitol dehydrogenase
- GP
Glycogen phosphorylase
- SP
Serine protease
- JHEH
Juvenile hormone epoxide hydrolase
- TRE
Trehalase
- PCNA
Proliferating cell nuclear antigen
- EST
Expressed sequence tag
Additional files
S1-Unigene expression. (XLSX 4.86 mb)
S2-Gene Ontology classification. (PDF 6.16 kb)
S3-Significantly enriched KEGG pathways in D vs. ND and DT vs. D. (XLSX 16.6 kb)
S4-Differentially expressed genes involved in fatty acid biosynthesis and cell cycle pathways. (XLSX 11.8 kb)
S5-Diapause-associated genes of interest. (XLSX 12.0 kb)
S6-The primer of qRT-PCR. (XLSX 10.3 kb)
Footnotes
Xiaoyang Qi and Lisheng Zhang contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The experiment was conceived and coordinated by XQ, LZ, YH, XR, JH, and HC in this study. XQ and LZ carried out the design of study, participated in the analysis of transcriptome data, and drafted the manuscript. XR and XQ participated in the sequence alignment and qPCR validation. YH participated in the collection of sampling. JH and HC conceived of the study, participated in its design and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Xiaoyang Qi, Email: qixiaoyang1024@163.com.
Lisheng Zhang, Email: zhangleesheng@163.com.
Yanhua Han, Email: hanyanhua_AYY@163.com.
Xiaoyun Ren, Email: renxiaoyunyouxiang@163.com.
Jian Huang, Email: jhuang1234@126.com.
Hongyin Chen, Email: hongyinc@163.com.
References
- 1.Nakamuta K, Saito T. Recognition of Aphid Prey by the Lady Beetle, Coccinella septempunctata bruckii Mulsant (Coleoptera: Coccinellidae) Appl Entomol Zool. 1985;20(4):479–83. [Google Scholar]
- 2.Gordon RD. The Coccinellidae (Coleoptera) of America north of Mexico. J N Y Entomol Soc. 1985;93:1–912. [Google Scholar]
- 3.Hodek I. The influence of Aphis sambuci L. as prey of the ladybird beetle Coccinella 7-punctata L. (In Czech, English abstract) Acta Soc Zool Bohemoslov. 1956;20:62–74. [Google Scholar]
- 4.Zhang LS, Chen HY, Li BP. Mass rearing and utilization of insect natural enemies (in Chinese) 1. Beijing: China Agriculture Scientech Press; 2014. [Google Scholar]
- 5.Zhu J, Park K. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J Chem Ecol. 2005;31(8):1733–46. doi: 10.1007/s10886-005-5923-8. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Zhang LS, Chen HY, Li YY, Zhang J. Research progress in diapause of the lady beetles (in Chinese) Chin J of Biol Control. 2011;37(5):27–33. [Google Scholar]
- 7.Hagen KS. Biology and ecology of predaceous Coccinellidae. Annu Rev Entomol. 1962;7(1):289–326. doi: 10.1146/annurev.en.07.010162.001445. [DOI] [Google Scholar]
- 8.Zeng FR, Chen HY. The system engineering for rearing insect natural enemies (in Chinese) 1. Beijing: China Agriculture Scientech Press; 2009. [Google Scholar]
- 9.Hodek I. Diapause development, diapause termination and the end of diapause. Eur J Entomol. 1996;93:475–87. [Google Scholar]
- 10.Tauber MJ, Tauber CA. Insect seasonality: diapause maintenance, termination, and postdiapause development. Annu Rev Entomol. 1976;21(1):81–107. doi: 10.1146/annurev.en.21.010176.000501. [DOI] [Google Scholar]
- 11.Tauber MJ, Tauber CA, Masaki S. Seasonal adaptations of insects. New York: Oxford University Press; 1986. [Google Scholar]
- 12.Sun Y, Wan FH. Quality control in mass rearing procedure of Coccinella septempunctata L. (Coleoptera: Coccinellidae) (in Chinese) Chin J of Biol Control. 2000;16(1):8–11. [Google Scholar]
- 13.Denlinger DL, Yocum GD, Rinehart JP. Hormonal control of diapause. In: Gilbert LI, Iatrou K, and Gill SS, editors. Comprehensive Insect Mole Sci. 2004;3:615–50. [Google Scholar]
- 14.Tanaka H, Sudo C, An Y, Yamashita T, Sato K, Kurihara M, et al. A specific peptide produced during adult diapause of the leaf beetle, Gastrophysa atrocyanea Motschulsky (Coleoptera: Chrysomelidae) Appl Entomol Zool. 1998;33(4):535–43. [Google Scholar]
- 15.Yoder JA, Denlinger DL. Water balance in flesh fly pupae and water vapor absorption associated with diapause. J Exp Biol. 1991;157:273–86. [Google Scholar]
- 16.Ikeda M, Su Z, Saito H, Imai K, Sato Y, Isobe M, et al. Induction of embryonic diapause and stimulation of ovary trehalase activity in the silkworm, Bombyx mori, by synthetic diapause hormone. J Insect Physiol. 1993;39(10):889–95. doi: 10.1016/0022-1910(93)90122-8. [DOI] [Google Scholar]
- 17.Noguchi H, Hayakawa Y. Dopamine is a key factor for the induction of egg diapause of the silkworm, Bombyx mori. Eur J Biochem. 2001;268(3):774–80. doi: 10.1046/j.1432-1327.2001.01933.x. [DOI] [PubMed] [Google Scholar]
- 18.Koštál V. Eco-physiological phases of insect diapause. J Insect Physiol. 2006;52(2):113–27. doi: 10.1016/j.jinsphys.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Denlinger DL. Regulation of diapause. Annu Rev Entomol. 2002;47(1):93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa K. The diapause hormone of the silkworm. Bombyx mori Nature. 1957;179:1300–1. doi: 10.1038/1791300b0. [DOI] [Google Scholar]
- 21.Dong Y, Desneux N, Lei C, Niu C. Transcriptome characterization analysis of Bactrocera minax and new insights into its pupal diapause development with gene expression analysis. Int J Biol Sci. 2014;10(9):1051–63. doi: 10.7150/ijbs.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poelchau MF, Reynolds JA, Denlinger DL, Elsik CG, Armbruster PA. A de novo transcriptome of the Asian tiger mosquito, Aedes albopictus, to identify candidate transcripts for diapause preparation. BMC Genomics. 2011;12:619. doi: 10.1186/1471-2164-12-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poelchau MF, Reynolds JA, Denlinger DL, Elsik CG, Armbruster PA. Transcriptome sequencing as a platform to elucidate molecular components of the diapause response in the Asian tiger mosquito Aedes albopictus. Physiol Entomol. 2013;38(2):173–81. doi: 10.1111/phen.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheolho S, Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc Natl Acad Sci U S A. 2008;105(18):6777–81. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai K, Konno T, Nakazawa Y, Komiya T, Isobe M, Koga K, et al. Isolation and structure of diapause hormone of the silkworm, Bombyx mori. Proceedings of the Japan Academy. Ser B: Phys Biol Sci. 1991;67(6):98–101. [Google Scholar]
- 26.Fukuda S, Takeuchi S. Diapause factor-producing cells in the suboesophageal ganglion of the silkworm, Bombyx mori L. Proc Japan Acad. 1967;43:51–6. doi: 10.1111/j.1440-169x.1967.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda S, Takeuchi S. Studies on the diapause factor‐producing cells in the suboesophageal ganglion of the silkworm, Bombyx mori L. Embryologia. 1967;9(4):333–53. doi: 10.1111/j.1440-169X.1967.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 28.Xu WH, Denlinger DL. Molecular characterization of prothoracicotropic hormone and diapause hormone in Heliothis virescens during diapause, and a new role for diapause hormone. Insect Mol Biol. 2003;12(5):509–16. doi: 10.1046/j.1365-2583.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Sun J, Zhang L, Shen J, Xu W. Cloning and expression of the cDNA encoding the FXPRL family of peptides and a functional analysis of their effect on breaking pupal diapause in Helicoverpa armigera. J Insect Physiol. 2004;50(1):25–33. doi: 10.1016/j.jinsphys.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai H, Kawai T, Takeda S. Physiological changes related to diapause of the lady beetle, Harmonia axyridis (Coleoptera: Coccinellidae) Appl Entomol Zool. 1992;27:479–87. [Google Scholar]
- 31.Berkvens N, Bonte J, Berkvens D, Tirry L, De Clercq P. Influence of diet and photoperiod on development and reproduction of European populations of Harmonia axyridis (Pallas)(Coleoptera: Coccinellidae) BioControl. 2008;53(1):211–21. doi: 10.1007/s10526-007-9130-0. [DOI] [Google Scholar]
- 32.Katsoyannos P, Kontodimas DC, Stathas GJ. Summer diapause and winter quiescence of Coccinella septempunctata (Col. Coccinellidae) in central Greece. Entomophaga. 1997;42(4):483–91. doi: 10.1007/BF02769808. [DOI] [Google Scholar]
- 33.Sakurai H, Goto K, Takeda S. Emergence of the ladybird beetle, Coccinella septempunctata bruckii Mulsant in the field. Res Bull Fac Agr Gifu Univ. 1983;48:37–45. [Google Scholar]
- 34.Wang W, Zhang LS, Chen HY, Wang J, Zhang J, Liu Y. Effects of temperature and light on Diapause induction in Lady Beetle Coccinella septempunctata in Beijing, China (in Chinese) Chin J of Biol Control. 2013;29(1):24–30. [Google Scholar]
- 35.Liu Y, Zhang LS, Chen HY, Huang FX, Jiang S, Ren XY. Differential expression of malate dehydrogenase and isocitrate dehydrogenase in diapaused ladybird, Coccinella septempunctata L (in Chinese) Chin J of Biol Control. 2014;30(5):593–9. [Google Scholar]
- 36.Ragland GJ, Denlinger DL, Hahn DA. Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc Natl Acad Sci U S A. 2010;107(33):14909–14. doi: 10.1073/pnas.1007075107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekblom R, Galindo J. Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity. 2011;107(1):1–15. doi: 10.1038/hdy.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu S, Wei W, Chu Y, Zhang L, Shen J, An C. De Novo transcriptome analysis of wing development-related signaling pathways in Locusta migratoria Manilensis and Ostrinia furnacalis (Guenée) PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0106770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ansorge WJ. Next-generation DNA, sequencing techniques. New Biotechnol. 2009;25(4):195–203. doi: 10.1016/j.nbt.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Schuster SC. Next-generation sequencing transforms today’s biology. Nat Methods. 2008;5(1):16–8. doi: 10.1038/nmeth1156. [DOI] [PubMed] [Google Scholar]
- 41.Vera JC, Wheat CW, Fescemyer HW, Frilander MJ, Crawford DL, Hanski I, et al. Rapid transcriptome characterization for a nonmodel organism using 454 pyrosequencing. Mol Ecol. 2008;17(7):1636–47. doi: 10.1111/j.1365-294X.2008.03666.x. [DOI] [PubMed] [Google Scholar]
- 42.Gibbons JG, Janson EM, Hittinger CT, Johnston M, Abbot P, Rokas A. Benchmarking next-generation transcriptome sequencing for functional and evolutionary genomics. Mol Biol Evol. 2009;26(12):2731–44. doi: 10.1093/molbev/msp188. [DOI] [PubMed] [Google Scholar]
- 43.Malone JH, Oliver B. Microarrays, deep sequencing and the true measure of the transcriptome. BMC Biol. 2011;9(1):34. doi: 10.1186/1741-7007-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Jiang R, Wu H, Liu P, Xie J, He Y, et al. Next-generation sequencing-based transcriptome analysis of Cryptolaemus montrouzieriunder insecticide stress reveals resistance-relevant genes in ladybirds. Genomics. 2012;100(1):35–41. doi: 10.1016/j.ygeno.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Tang L, Wang X, Jin F, Qiu B, Wu J, Ren S. De Novo sequencing-based transcriptome and digital gene expression analysis reveals insecticide resistance relevant genes in Propylaea japonica (Thunberg) (Coleoptea: Coccinellidae) PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0100946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Etebari K, Palfreyman RW, Schlipalius D, Nielsen LK, Glatz RV, Asgari S. Deep sequencing-based transcriptome analysis of Plutella xylostella larvae parasitized by Diadegma semiclausum. BMC Genomics. 2011;12(1):446. doi: 10.1186/1471-2164-12-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y, Zhou W, Zhou Y, Wu J, Zhou X. Transcriptome and comparative gene expression analysis of Sogatella furcifera (Horváth) in response to Southern rice black-streaked dwarf virus. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0036238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kappe SH, Gardner MJ, Brown SM, Ross J, Matuschewski K, Ribeiro JM, et al. Exploring the transcriptome of the malaria sporozoite stage. Proc Natl Acad Sci U S A. 2001;98(17):9895–900. doi: 10.1073/pnas.171185198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranz JM, Castillo-Davis CI, Meiklejohn CD, Hartl DL. Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science. 2003;300(5626):1742–5. doi: 10.1126/science.1085881. [DOI] [PubMed] [Google Scholar]
- 50.Domazet-Lošo T, Tautz D. A phylogenetically based transcriptome age index mirrors ontogenetic divergence patterns. Nature. 2010;468(7325):815–8. doi: 10.1038/nature09632. [DOI] [PubMed] [Google Scholar]
- 51.Ali MR, Lim J, Kim Y. Transcriptome of a specialized extra‐embryonic cell, teratocyte, and its host immunosuppressive role revealed by ex vivo RNA interference. Insect Mol Biol. 2015;24(1):13–28. doi: 10.1111/imb.12132. [DOI] [PubMed] [Google Scholar]
- 52.Jaeger PA, Doherty C, Ideker T. Modeling transcriptome dynamics in a complex world. Cell. 2012;151(6):1161–2. doi: 10.1016/j.cell.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 53.Hodek I, Ruzicka Z. Photoperiodic response in relation to diapause in Coccinella septempunctata (Coleoptera) [biological control of aphids] Acta Entomol Bohemoslov. 1979;76:209–18. [Google Scholar]
- 54.De Wilde J, Duintjer CS, Mook L. Physiology of diapause in the adult Colorado beetle (Leptinotarsa decemlineata Say)—I The photoperiod as a controlling factor. J Insect Physiol. 1959;3(2):75–85. doi: 10.1016/0022-1910(59)90022-8. [DOI] [Google Scholar]
- 55.Kimura MT. Quantitative response to photoperiod during reproductive diapause in the Drosophila auraria species-complex. J Insect Physiol. 1990;36(3):147–52. doi: 10.1016/0022-1910(90)90115-V. [DOI] [Google Scholar]
- 56.Wang W. Effects of temperature and photoperiod on regulation of diapause and post-diapause biology in Coccinella septempunctata (in Chinese) Chinese Academy of Agricultural Sceiences: Beijing; 2012. [Google Scholar]
- 57.Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;43:222–6. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hahn DA, Denlinger DL. Meeting the energetic demands of insect diapause: nutrient storage and utilization. J Insect Physiol. 2007;53(8):760–73. doi: 10.1016/j.jinsphys.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 59.Rinehart JP, Yocum GD, Denlinger DL. Developmental upregulation of inducible hsp70 transcripts, but not the cognate form, during pupal diapause in the flesh fly, Sarcophaga crassipalpis. Insect Biochem Mol Biol. 2000;30(6):515–21. doi: 10.1016/S0965-1748(00)00021-7. [DOI] [PubMed] [Google Scholar]
- 60.Rinehart JP, Denlinger DL. Heat-shock protein 90 is down-regulated during pupal diapause in the flesh fly, Sarcophaga crassipalpis, but remains responsive to thermal stress. Insect Mol Biol. 2000;9(6):641–5. doi: 10.1046/j.1365-2583.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 61.Roeder A, Kirschning CJ, Rupec RA, Schaller M, Korting HC. Toll-like receptors and innate antifungal responses. Trends Microbiol. 2004;12(1):44–9. doi: 10.1016/j.tim.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Samanta M, Swain B, Basu M, Panda P, Mohapatra GB, Sahoo BR, et al. Molecular characterization of toll-like receptor 2 (TLR2), analysis of its inductive expression and associated down-stream signaling molecules following ligands exposure and bacterial infection in the Indian major carp, rohu (Labeo rohita) Fish Shellfish Immun. 2012;32(3):411–25. doi: 10.1016/j.fsi.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 63.Bateman RL, Bhanumoorthy P, Witte JF, McClard RW, Grompe M, Timm DE. Mechanistic Inferences from the Crystal Structure of Fumarylacetoacetate Hydrolase with a Bound Phosphorus-based Inhibitor. J Biol Chem. 2001;276(18):15284–91. doi: 10.1074/jbc.M007621200. [DOI] [PubMed] [Google Scholar]
- 64.Okuda T, Tanaka S, Kotaki T, Ferenz HJ. Role of the corpora allata and juvenile hormone in the control of imaginal diapause and reproduction in three species of locusts. J Insect Physiol. 1996;42(10):943–51.
- 65.de Kort CAD, Koopmanschap AB, Strambi C, Strambi A. The application and evaluation of radioimmunoassay for measuring juvenile hormone titres in Colorado beetle hemolymph. Insect Biochem. 1985;15(6):771–5. doi: 10.1016/0020-1790(85)90106-4. [DOI] [Google Scholar]
- 66.de Kort CAD, Bergot BJ, Schooley DA. The nature and titre of juvenile hormone in the Colorado potato beetle, Leptinotarsa decemlineata. J Insect Physiol. 1982;28(5):471–4. doi: 10.1016/0022-1910(82)90077-4. [DOI] [Google Scholar]
- 67.de Kort CAD. Thirty-five years of diapause research with the Colorado potato beetle. Entomol Exp Appl. 1990;56(1):1–13. doi: 10.1111/j.1570-7458.1990.tb01376.x. [DOI] [Google Scholar]
- 68.Shiga S, Hamanaka Y, Tatsu Y, Okuda T, Numata H . Juvenile hormone biosynthesis in diapause and nondiapause females of the adult blow fly Protophormia terraenovae. Zoolog Sci. 2009;20(10):1199–206. [DOI] [PubMed]
- 69.Niimi T, Yamashita O, Yaginuma T. A cold-inducible Bombyx gene encoding a protein similar to mammalian sorbitol dehydrogenase. Eur J Biochem. 1993;213(3):1125–31. doi: 10.1111/j.1432-1033.1993.tb17862.x. [DOI] [PubMed] [Google Scholar]
- 70.Yamashita O, Hasegawa K, Seki M. Effect of the diapause hormone on trehalase activity in pupal ovaries of the silkworm, Bombyx mori L. Gen Comp Endocrinol. 1972;18(18):515–23. doi: 10.1016/0016-6480(72)90031-7. [DOI] [PubMed] [Google Scholar]
- 71.Su ZH, Ikeda M, Sato Y, Saito H, Imai K, Isobe M, et al. Molecular characterization of ovary trehalase of the silkworm, Bombyx mori and its transcriptional activation by diapause hormone. Biochim Biophys Acta. 1994;1218(3):366–74. doi: 10.1016/0167-4781(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 72.Robich RM, Denlinger DL. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. PNAS. 2005;102(44):15912–7. doi: 10.1073/pnas.0507958102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tammariello SP. G0/G1 cell cycle arrest in the brain of Sarcophaga crassipalpis during pupal diapause and the expression pattern of the cell cycle regulator, proliferating cell nuclear antigen. Insect Biochem Molec Biol. 1998;28(2):83–9. doi: 10.1016/S0965-1748(97)00082-9. [DOI] [PubMed] [Google Scholar]
- 74.Kramer JM, Davidge JT, Lockyer JM, Staveley BE. Expression of Drosophila FOXO regulates growth and can phenocopy starvation. BMC Dev Biol. 2003;3:5. doi: 10.1186/1471-213X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang LS, Chen HY. Patent ZL. 2012 1 0279680. 6, issued March 26, 2014 (in Chinese).
- 76.Zhang LS, Chen HY, Wang W, Wang MQ, Liu CX, Wang SY, et al. Patent ZL. 2012 1 0279647. 3, issued March 26, 2014 (in Chinese).
- 77.FASTX-Toolkit software package, http://hannonlab.cshl.edu/fastx_toolkit/ . Accessed 09 February 2015.
- 78.Su C, Chao Y, Chang YA, Chen W, Chen C, Lee A, et al. De novo assembly of expressed transcripts and global analysis of the Phalaenopsis aphrodite transcriptome. Plant Cell Physiol. 2011;52(9):1501–14. doi: 10.1093/pcp/pcr097. [DOI] [PubMed] [Google Scholar]
- 79.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 80.KAAS, KEGG automatic annotation server. http://www.genome.jp/tools/kaas/. Accessed 11 February 2015.
- 81.Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 82.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5(7):621–8. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]


