Figure 1. Comparison of the toxic principles of PrPS c and tau.
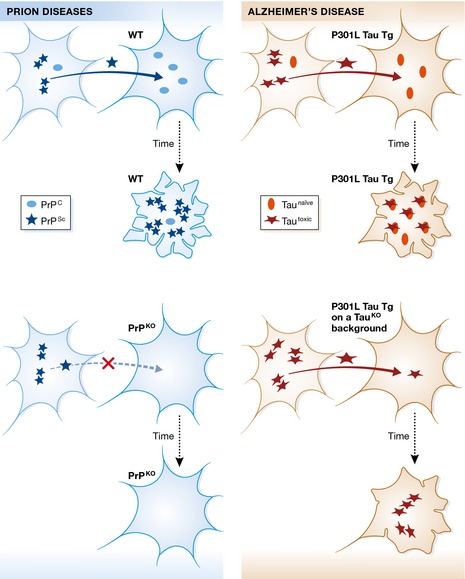
The present report addresses what is known about the propagation and toxicity of prions (shown on the left) and asks the question whether tau in Alzheimer's disease (on the right) displays the same characteristics. In the case of prion diseases, a form of the prion protein known as PrPS c (Scrapie) propagates from cell to cell and, upon uptake by a recipient cell, leads to an infectious conversion of the endogenous, cellular form of prion (PrPC) into the toxic PrPS c form, ultimately leading to neuronal demise (as graphically illustrated by the extremely ruffled cell membrane). Importantly, both propagation and toxicity are halted, when the recipient cells lacks endogenous PrPC expression (PrP knockout, PrPKO). In their study, Wegmann et al (2015) use different transgenic (P301L Tau Tg) and viral paradigms, to demonstrate that, although P301L tau still propagates, there is a decrease in its misfolding and neurotoxicity in the absence of endogenous mouse tau (as visualized by the different extent of membrane ruffling under the two conditions). The assumption is that P301L tau is synonymous with tautoxic and that endogenous mouse tau exists in a naïve form. As a possible model to explain the reduced toxicity in the absence of endogenous (naïve) tau, the authors suggest that co‐aggregation of mouse tau with human P301L tau may create more toxic “strains” of tau aggregates, with stronger effects on tau pathology in mice. The authors conclude that P301L mutant tau may not be a genuine prion.
