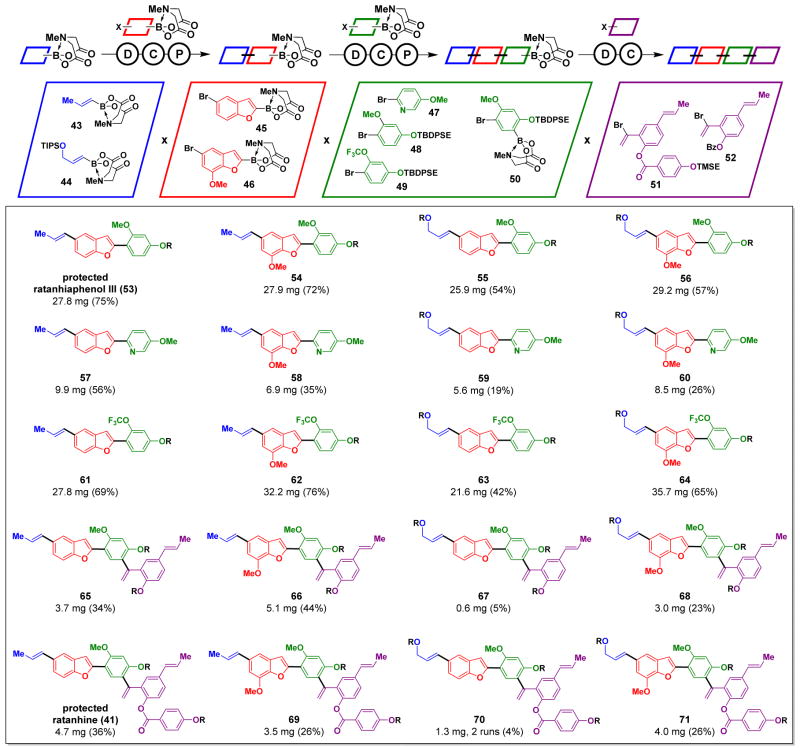
Fig. 3. Automated synthesis of ratanhine derivatives.
Conditions: deprotection – NaOH, THF:H2O; coupling – cycle 1: Pd(OAc)2, SPhos, K2CO3, THF, 55 °C, 16h. cycle 2: Pd(OAc)2, XPhos, K3PO4, THF, 55 °C, 14 h. cycle 3: Pd(OAc)2, SPhos, K3PO4, THF, 55 °C, 24 h; purification – SiO2, MeOH:Et2O; THF. All protecting groups other than MIDA (R = TIPS, TBDPSE, TMSE, or Bz) were successfully removed in a separate step (24).