Summary
Follicular T helper (Tfh) cells have a crucial role in regulating immune responses within secondary lymphoid follicles by directing B cell differentiation towards memory B cells and plasma cells. Because abnormal humoral responses are key features in both primary Sjögren's syndrome (pSS) and systemic lupus erythematosus (SLE), the aim of this study was to profile the pathological connection between peripheral Tfh cells and B cells in the two diseases. Twenty‐five pSS patients, 25 SLE patients and 21 healthy controls were enrolled into the study. We determined the ratio of circulating Tfh‐like cells, their interleukin (IL)‐21 production and different B cell subsets by flow cytometry. We observed higher percentages of naive B cells in both diseases, while non‐switched and switched memory B cells showed decreased frequencies. The proportions of double‐negative B cells and plasmablasts were elevated in SLE and decreased in pSS. The percentages of transitional B cells and mature‐naive B cells were higher in SLE. Patients with more severe disease course had an elevated ratio of TFH‐like cells and increased IL‐21 production. Moreover, expansion of Tfh‐like cells correlated positively with parameters related to antibody secretion, including serum immunoglobulin (Ig)G, immune complexes (ICs) and autoantibodies. Correlation analysis between Tfh‐like cells and certain B cell subsets revealed possible defects during B cell selection. In conclusion, our observations on the profound expansion of circulating Tfh‐like cells and their IL‐21 production, along with the characteristic aberrant peripheral B cell distribution in both pSS and SLE, indicate the prominent role of Tfh cell in the regulation of B cell selection.
Keywords: B cell, follicular T helper cell, interleukin‐21, primary Sjögren's syndrome, systemic lupus erythematosus
Introduction
Autoimmune diseases are characterized by the breakdown of immune tolerance leading to autoreactive immune mechanisms and consequential tissue and organ damages. In the pathogenesis of autoimmune diseases, various cellular and humoral immune processes have been described, including disproportional T and B cell responses, altered cytokine milieu and disturbed apoptotic processes, which result in exaggerated immune responses. In the last decades, a large amount of studies confirmed that B cell activation plays a crucial role in the inflammatory processes through antigen presentation, autoantibody production and secretion of numerous proinflammatory factors. However, besides the established disease‐promoting role of B cells, certain B cell subsets, so‐called regulatory B (Breg) cells have a negative regulatory effect by producing regulatory cytokines such as interleukin (IL)‐10, and interacting directly with activated T cells via cell‐to‐cell contact 1.
The proliferation and differentiation of B cells depend highly upon their collaboration with a special subset of CD4+ T cells in the germinal centres (GCs) of secondary lymphoid organs. These recently described T cells, so‐called follicular T helper (Tfh) cells, are generated from peripheral naive CD4+ T cells in the T cell zone of lymphoid organs. The proper interplay of activated B cells and Tfh cells is essential for the development of extrafollicular short‐lived, low‐affinity plasma cells and also for GC responses. Within GCs, Tfh cells facilitate the generation of high‐affinity memory B cells and long‐lived plasma cells. IL‐21 is the hallmark cytokine of Tfh cells and has been shown to function as a potent inducer of plasma cell formation; moreover, it is also involved in GC B cell selection 2.
Considering the critical role of Tfh cells and their IL‐21 cytokine secretion in B cell activation and antibody production, their failure to maintain self‐tolerance and potential contribution to autoimmunity drew intensive attention. Recent investigations shed light on altered Tfh profiles in various autoimmune conditions. In systemic lupus erythematosus (SLE), which is a prototypical systemic autoimmune disease characterized by immune complex‐mediated systemic tissue damage, it is well established that the imbalance of different subsets of B cells is crucial for the initiation and perpetuation of the disease. Recently, elevated percentages of peripheral CD4+ inducible T cell co‐stimulator (ICOS)high and CD4+CXCR5+ICOShigh Tfh cells were reported in SLE patients. Notably, these cell proportions showed associations with autoantibody titres and the presence of glomerulonephritis 3. Moreover, corticosteroid pulse therapy down‐regulated the number of circulating Tfh cells, indicating that Tfh cells may be good therapeutic targets in the disease 4.
In primary Sjögren's syndrome (pSS), which is a common systemic autoimmune disease characterized by chronic inflammation and consequential destruction of exocrine glands, humoral autoimmune responses, B cell activation and autoantibody production are also key immune abnormalities. Immunohistological analysis of biopsies from minor salivary glands commonly demonstrates the presence of ectopic GCs in pSS. The number of GCs in salivary glands correlates with the severity of inflammation, and anti‐Sjögren's‐syndrome‐related antigen A (SSA)/Ro and anti‐SSB/La autoantibody production. Moreover, the formation of ectopic GCs carries a higher risk of developing B cell lymphoma 5. Recently, we demonstrated elevated circulating CD4+CXCR5+ICOS+programmed death 1 (PD‐1)+ Tfh cell percentages in pSS. Based on our data, we found a strong association between the Tfh cell proportions and the presence of systemic extraglandular manifestations (EGMs) and anti‐SSA/SSB autoantibody positivity. Patients with higher Tfh cell proportions also had elevated serum levels of IL‐12 and IL‐21 6. Maehara et al. have described that the expression of Th2 and certain Tfh‐related molecules was associated with robust lymphocytic accumulation and ectopic GC formation 7. We also investigated the localization of Tfh cells in the lymphocyte infiltration in labial salivary gland (LSG) biopsies gained from pSS patients at the time of disease onset, and found that Tfh cell markers [CD84, PD‐1 and B cell lymphoma 6 protein (Bcl‐6)] occurred predominantly in more organized lymphoid structures with higher focus scores 8, which indicates that Tfh cells may also be good therapeutic targets in pSS.
However, the complex interplay between Tfh cells and the special subsets of B cells has not yet been elucidated fully. Because identification of the pathogenic pathways and the corresponding biomarkers linking abnormal cellular activity to disease activity and outcome is essential for defining proper therapeutic targets, the aim of the present study was to profile the pathological connection between the peripheral Tfh cells and B cell subsets and the clinical features of pSS and SLE.
Materials and methods
Patients and healthy individuals
A total of 25 patients with pSS (24 female and one male; mean age: 57·88 ± 9·19 years) and 25 patients with SLE (24 female and one male; mean age: 41·17 ± 13·20 years) were enrolled into the study. All patients were recruited from the out‐patient clinic for systemic autoimmune diseases at the Division of Clinical Immunology, University of Debrecen, where they received regular follow‐up treatment. The average disease duration in case of pSS was 13·40 ± 7·94 years, while 11·44 ± 9·14 years in SLE. The diagnosis of pSS was based on the European–American consensus criteria 9. Among pSS patients, 15 suffered from EGMs, while 10 had only glandular symptoms. The distribution of EGMs of pSS patients were as follows: polyarthralgia n = 11, Raynaud's phenomenon n = 7, polyarthritis n = 6 and vasculitis n = 2. The exclusion criteria included therapy with immunosuppressive/immunomodulant agents. Vasculitis or other EGMs needing immunosuppressive treatment were newly recognized. All patients with SLE fulfilled the corresponding diagnostic criteria for lupus 10, 11, and their disease activity was assessed by the SLE Disease Activity Index (SLEDAI). SLE patients were classified according to having inactive or active disease status: the SLEDAI < 6 group comprised subjects with inactive disease (n = 17), while SLEDAI ≥ 6 group consisted of subjects with active disease (n = 8). All the SLE patients received methylprednisolone therapy per os with an average dose of 4 mg daily; the dose of the treatment did not exceed 8 mg methylprednisolone per day in any case of SLE patients. The blood samples were collected from SLE patients 24 h after taking the regular methylprednisolone medication. The active disease status in the corresponding SLE patients was newly recognized, and these patients had undergone the blood sampling before any changes in their therapy. In addition, patients were also categorized according to their serological markers with a special emphasis on the presence of autoantibodies. Among pSS patients, we found nine anti‐Ro/SSA positive (SSA > 10 U/ml) individuals, four of whom were anti‐Ro/SSA–anti‐La/SSB double‐positive. Regarding SLE patients, 15 of them were anti‐dsDNA‐positive (dsDNA > 20 IU/ml). The control group consisted of 21 age‐ and sex‐matched (20 female and one male; mean age: 39·10 ± 12·43 years) healthy volunteers. No patients or controls enrolled into this study had ongoing infections, either viral or bacterial. Informed written consent was obtained from all subjects, and the study was approved by the Ethics Committee of the University of Debrecen. All experiments carried out were in compliance with the Declaration of Helsinki.
Cell surface staining and flow cytometric analysis
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood sample by Ficoll‐Histopaque (Sigma‐Aldrich, St Louis, MO, USA) density‐gradient centrifugation. Cells were then harvested and washed twice and stained for 20 min at 4°C using specific antibodies. For identification of naive and memory B cell subsets, we used IgD‐fluorescein isothiocyanate (FITC) (clone: IADB6)/CD27‐phycoerythrin (PE) (clone: 1A4CD27)/CD19‐phycoerythrin‐cyanin dye 5 (PE‐Cy5) (clone: J3‐119) (all from Beckman Coulter Inc., Fullerton, CA, USA and Immunotech, Marseille, France). To identify naive, mature‐naive, primarily memory and transitional B cell subpopulations, cells were stained with the following combination of monoclonal antibodies: CD38‐FITC (clone: HIT2)/CD27‐PE/CD19‐PE‐Cy5/CD24‐allophycocyanin (APC) (ML5) (BD Biosciences, San Diego, CA, USA and Beckman Coulter and BioLegend, San Diego, CA, USA). At least 20 000 CD19+ events of each sample were analysed within the whole lymphocyte population. For the assessment of circulating Tfh cells we used CXCR5‐Alexa Fluor 488 (clone: RF8B2)/ICOS‐PE (clone: DX29)/PD‐1‐peridinin‐chlorophyll protein‐cyanin dye 5·5 (PerCP‐Cy5·5) (clone: EH12·1)/CD4‐APC (clone: RPA‐T4) (all from BD Biosciences) monoclonal antibodies. For the determination of naive and activated or memory CD4+ T cell subsets we used CD45RA‐PE (clone: HI100) and CD45RA‐PerCP‐Cy5·5 (clone: HI100) monoclonal antibodies (both from BioLegend). Fluorescence Minus One controls were used in all procedures. The stained cells were measured by fluorescence activated cell sorter (FACS)Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) and data were analysed using FlowJo Software (Treestar, Ashland, OR, USA). In case of Tfh cells at least 75 000 CD4+ events per sample were analysed within the whole lymphocyte population.
Intracellular cytokine analysis by flow cytometry
The cytoplasmic IL‐21 content of circulating Tfh cells was also determined by flow cytometry. Briefly, isolated PBMCs were cultured in modified RPMI‐1640 medium with GlutamaxTM‐I (Life Technologies Corporation, Carlsbad, CA, USA) supplemented with 100 U/ml penicillin, 100 ng/ml streptomycin and 10% heat‐inactivated fetal calf serum (Life Technologies) at a concentration of 2 × 106/ml in 24‐well tissue culture plates for analysing single‐cell cytokine production. Total PBMCs were incubated with phorbol‐12‐myristate 13‐acetate (PMA) (25 ng/ml), ionomycin (1 μg/ml) and Golgi Stop brefeldin A (10 μg/ml) (all from Sigma Aldrich) for 5 h at 37°C in 5% CO2 milieu. Cell surface staining was performed with a cocktail of CXCR5‐AF488, PD‐1‐PerCP‐Cy5·5 and CD4‐APC monoclonal antibodies for 20 min at 4°C. The cells were then fixed and permeabilized with IntraprepTM permeabilization reagent (Beckman Coulter Inc, Miami, FL, USA), according to the manufacturer's instructions, and intracellular cytokines were stained with anti‐IL‐21‐PE (clone: 3A3‐N2·1) (BD Biosciences).
For intracellular IL‐10 induction of B cells, PBMCs were stimulated with cytosine–phosphate–guanosine (CpG) (Toll‐like receptor 9 ligand, ODN 2006 type B; 0·5 μM/ml; Hycult Biotech Inc., Uden, the Netherlands) for 48 h at 37°C in 5% CO2 milieu. For the last 5 h, PMA (25 ng/ml), ionomycin (1 μg/ml) and Golgi Stop brefeldin A (10 μg/ml) were added to the culture. Cells were then harvested, washed in staining buffer and incubated for 20 min at 4°C with CD19‐PE‐Cy5. Intracellular staining method was performed with IL‐10‐PE (clone: JES3‐9D7), as described above. Measurements were performed and data were collected by FACSCalibur flow cytometer and data were analysed using FlowJo software. At least 75 000 CD4+ events or 15 000 CD19+ events of each sample were analysed within the entire lymphocyte population. The viability of the CpG exposed cells were determined by 7‐aminoactinomycin‐D (7‐AAD; BioLegend) staining with flow cytometric analysis. The identification of B cells was performed with CD19‐FITC (clone J3‐119) monoclonal antibody. The percentages of dead cells among the CD19+ B cells were increased in the phorbol PMA + ionomycin + brefeldin A (PIB) and CpG + PIB group; however, the average percentages of dead cells remained < 2·5%. Regarding the whole lymphocyte group, the proportions of dead cells did not exceed 5% on average (Fig. S1).
Assessment of anti‐dsDNA, anti‐ro/SSA and anti‐la/SSB autoantibodies
As part of the routine diagnostic evaluation, autoantibodies were determined by enzyme‐linked immunosorbent assay (ELISA) technique with AUTOSTAT II kits (Hycor Biomedical, Indianapolis, IN, USA), according to the manufacturer's instructions.
Statistical analysis
Data were represented and statistical analysed with GraphPad Prism version 5 software (Graphpad Software, San Diego, USA). Data are presented as mean ± standard deviation (s.d.). To assess the distribution of the data, the Shapiro–Wilk normality test was used. In cases of normal distribution, if the F probe was granted we used the unpaired t‐test; if it was not granted we used the unpaired t‐test with Welch's correction for statistical comparison of the experimental data. In cases where distributions of the data set was different from normal, the Mann–Whitney U‐test was used. The correlations between two variables were evaluated with Pearson's correlation coefficient, while in cases of non‐normal distribution, Spearman's test was used. Differences were considered statistically significant at P < 0·05.
Results
The distribution of peripheral B cell subpopulations
According to the expression of IgD, CD27, CD38 and CD24 cell surface markers, the following B cell subsets were identified: CD19+IgD+CD27− naive B cells, CD19+IgD+CD27+ non‐switched memory B cells, CD19+IgD−CD27+ switched memory B cells, CD19+IgD−CD27− double‐negative (DN) B cells, CD19+CD38−CD24hiCD27+ primarily memory B cells, CD19+CD38hiCD24hiCD27− transitional B cells, CD19+CD38+CD24+ mature‐naive B cells and CD19+CD38hiCD27hi plasmablasts. Cells were quantified as their percentage in the CD19+ lymphocyte population.
Regarding CD19+IgD−CD27+ B cells, percentages were decreased in the overall pSS patient population compared to values in healthy individuals (15·07 ± 7·65% versus 23·23 ± 6·78%, respectively, P = 0·0005) (Fig. 1a). Correspondingly, both subgroups of pSS patients had significantly lower percentages compared to controls (pSS without EGMs versus control: 17·30 ± 5·06% versus 23·23 ± 6·78%, respectively, P = 0·0207; pSS with EGMs versus control: 13·58 ± 8·83% versus 23·23 ± 6·78%, respectively, P = 0·0007) (Fig. 1a). On the contrary, CD19+IgD−CD27+ B cell percentages in the total SLE patient population and controls were similar. However, SLE patients with SLEDAI < 6, unlike patients with SLEDAI > 6, had significantly decreased CD19+IgD−CD27+ B cell percentages compared to controls (17·53 ± 13·89% versus 23·23 ± 6·78%, respectively, P = 0·0321).
Figure 1.

Comparative analysis of peripheral B cell subsets from primary Sjögren's syndrome (pSS) patients, systemic lupus erythematosus (SLE) patients and healthy individuals. Peripheral blood mononuclear cells (PBMCs) were isolated from 25 pSS patients, 25 SLE patients and 21 healthy controls then stained with labelled antibodies as described previously. Peripheral blood B cell subsets were quantified as their percentage in CD19+ lymphocyte population. Representative immunoglobulin (Ig)D–CD27 and CD38–CD24 dot‐plots indicating the distribution and percentages of different B cell subsets. (a) Percentages of CD19+IgD−CD27+ switched‐memory B cells in the overall pSS patient population (n = 25), pSS with only glandular symptoms (n = 10), pSS with: extraglandular manifestations (EGMs) (n = 15), total SLE patient group (n = 25), SLE with SLE Disease Activity Index (SLEDAI) < 6 (n = 17), SLE with SLEDAI > 6 (n = 8) and control subjects (n = 21). (b) Frequency of CD19+IgD+CD27+ non‐switched memory B cells. (c) Proportions of CD19+IgD−CD27− double negative (DN) B cells. (d) Ratio of CD19+IgD+CD27− naive B cells. (e) Distribution of CD19+CD38−CD24hiCD27+ primarily memory B cells. (f) Percentages of CD19+CD38hiCD24hiCD27− transitional B cells. (g) Proportion of CD19+CD38+CD24+ mature‐naive B cells. (h) Frequency of CD19+CD38hiCD27hi plasmablasts. Each data point represents an individual subject, horizontal lines show the mean values with standard deviation (s.d.). Statistically significant differences are indicated by *P < 0·05; **P < 0·01; *** P < 0·001; ****P < 0·0001.
The ratio of CD19+IgD+CD27+ B cells was significantly lower in the whole SLE group than in healthy individuals (10·98 ± 10·60% versus 21·64 ± 11·52%, respectively, P = 0·0019) (Fig. 1b). This observation was also valid for SLEDAI < 6 and SLEDAI > 6 patient subgroups separately (13·21 ± 11·88% versus 21·64 ± 11·52%, respectively, P = 0·0333 and 6·25 ± 5·03% versus 21·64 ± 11·52%, respectively, P < 0·0001) (Fig. 1b).
Peripheral CD19+IgD−CD27−DN B cell proportions were reduced significantly in the overall pSS patient group set against control values (3·214 ± 2·463% versus 3·796 ± 1·681%, respectively, P = 0·0290). However, intragroup differences could be found only in pSS patients with EGMs when compared to controls (3·306 ± 3·030% versus 3·796 ± 1·681%, respectively, P = 0·0291) (Fig. 1c). Interestingly, in contrast to measurements in pSS, the percentages of CD19+IgD−CD27− DN B cells were heightened significantly in the whole SLE group than in healthy individuals (6·906 ± 4·525% versus 3.796 ± 1.681%, respectively, P = 0.0119). Nevertheless, this significant change could be detected only in SLEDAI < 6 patient subgroup compared to controls (7·017 ± 4·998% versus 3·796 ± 1·681%, respectively, P = 0·0204) (Fig. 1c).
The percentages of CD19+IgD+CD27− B cells were increased significantly in the whole pSS patient group compared to controls (63·87 ± 20·76% versus 51·32 ± 15·14%, respectively, P = 0·0261); however, this significant elevation could be observed only in pSS patients without EGMs (63·70 ± 14·14% versus 51·32 ± 15·14%, respectively, P = 0·0382) (Fig. 1d). Frequency of CD19+IgD+CD27− B cells was also elevated significantly in the total SLE patient group (62·88 ± 21·87% versus 51·32 ± 15·14%, respectively, P = 0.0471) (Fig. 1d). This tendency was also found in the remaining patient subgroups; however, the differences were not significant.
The percentages of CD19+CD38−CD24hiCD27+ B cell ratio were decreased significantly in pSS patients without EGMs when compared to healthy subjects (23·29 ± 9·827% versus 34·58 ± 13·91%, respectively, P = 0·0289) (Fig. 1e); of note, this difference was observed between pSS with EGMs and healthy subjects. The frequency of CD19+CD38−CD24hiCD27+ B cells was reduced significantly in the whole SLE patient group and SLEDAI > 6 group, compared to control values (22·77 ± 14·45% versus 34·5 ± 13·91%, respectively, P = 0·0074 and 17·10 ± 12·11% versus 34·58 ±13·91%, respectively, P = 0·0042) (Fig. 1e).
There was no difference in the pSS groups regarding the distribution of both CD19+CD38hiCD24hiCD27− and CD19+CD38+CD24+ B cell subsets. However, this could be due to the low number of patients in the subdivided groups. Conversely, we found that the ratio of CD19+CD38hiCD24hiCD27− B cells was elevated significantly in pSS patients with the presence of anti‐Ro/SSA antibody compared to subjects in whom autoantibody levels were under the threshold value (9·842 ± 7·766% versus 3·722 ± 2·320%, respectively, P = 0·0499) (data not shown). In the overall SLE group and in patients with SLEDAI > 6, the frequency of CD19+CD38hiCD24hiCD27− B cells was significantly higher than control values (10·35 ± 7·78% versus 5·30 ± 2·42%, respectively, P = 0·0045 and 12·71 ± 7·73% versus 5·30 ± 2·42%, respectively, P = 0·0323) (Fig. 1f). Furthermore, the ratio of CD19+CD38+CD24+ B cells was also elevated significantly not only in the total SLE group, but in the SLEDAI > 6 group compared to those measured in healthy subjects (35·86 ± 14·86% versus 27·74 ± 9·33%, respectively, P = 0·0297 and 39·32 ± 11·61% versus 27·74 ± 9·33%, respectively, P = 0·0094) (Fig. 1f).
The ratio of peripheral CD19+CD38hiCD27hi plasmablasts showed a different tendency in the two diseases. The percentages of these cells were significantly lower in the overall group of pSS patients, as well as in the patients with EGMs groups than control values (0·134 ± 0·154% versus 0·267 ± 0·293%, respectively, P = 0·0050 and 0·127 ± 0·183% versus 0·267 ± 0·293%, respectively, P = 0·0014) (Fig. 1h). However, in patients with SLE, we found a significantly higher frequency of CD19+CD38hiCD27hi plasmablasts only in the SLEDAI > 6 group compared to healthy subjects (1·150 ± 1·181% versus 0·2671 ± 0·2932%, respectively, P = 0·0403) (Fig. 1h).
Quantification of circulating Tfh‐like cells
Circulating Tfh‐like cells were quantified as their percentages within the CD4+ lymphocytes of peripheral blood. Within CD4+CXCR5+ lymphocytes, we also determined the fraction of ICOS+PD‐1+ T cells. According to our results, the percentages of CD4+CXCR5+ICOS+PD‐1+ Tfh‐like cells were increased significantly in pSS patients with EGMs when compared to pSS patients with glandular symptoms and healthy controls (0·4121 ± 0·2753% versus 0·2253 ± 0·1238%, respectively, P = 0·0322 and 0·4121 ± 0·2753% versus 0·2235 ± 0·0979%, respectively, P = 0·0218) (Fig. 2a). The frequency of Tfh‐like cells was also elevated in SLE; however, this difference was significant only when the overall SLE patients were compared with the controls (0·3369 ± 0·2029% versus 0·2235 ± 0·0979%, respectively, P = 0·0184) (Fig. 2a). Additionally, we analysed the distribution of Tfh cell percentages in the patient groups according to the absence or presence of specific autoantibodies. The ratio of Tfh cells showed a significant twofold increase in the anti‐Ro/SSA antibody‐positive group compared to healthy controls and the anti‐Ro/SSA antibody‐negative group (0·4806 ± 0·2699% versus 0·2235 ± 0·0979%, respectively, P = 0·0050 and 0·4806 ± 0·2699% versus 0·2568 ± 0·1893%, respectively, P = 0·0138) (Fig. 2b). Regarding the anti‐dsDNA antibody‐positive group, we found a significant 1·6‐fold increase compared to control values (0·3717 ± 0·2153% versus 0·2235 ± 0·0979%, respectively, P = 0·0192) (Fig. 2b).
Figure 2.
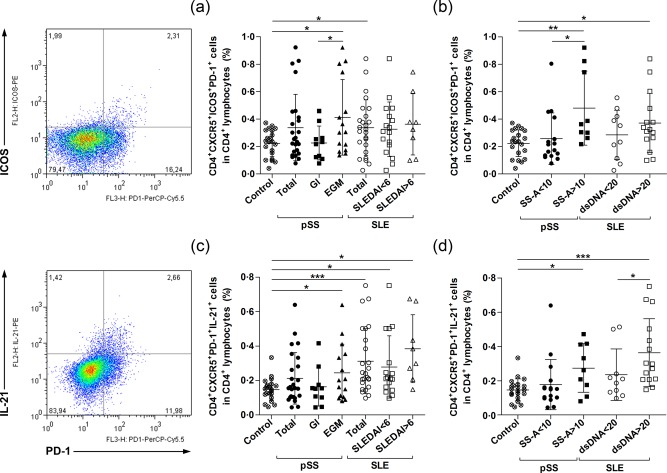
Enumeration of circulating follicular T helper cells (Tfh)‐like cells in primary Sjögren's syndrome (pSS) patients, systemic lupus erythematosus (SLE) patients and healthy subjects. Peripheral blood mononuclear cells (PBMCs) were isolated from 25 pSS patients; 25 SLE patients and 21 healthy controls were then stained with labelled antibodies as described previously. Circulating Tfh‐like cells were assessed as their percentage in the CD4+ lymphocyte population. Representative dot‐plots are shown the frequency of ICOS+programmed death 1 (PD‐1)+ and PD‐1+interleukin (IL)‐21+ cells within the CD4+CXCR5+ cell population. (a) Percentages of CD4+CXCR5+ICOS+PD‐1+ Tfh‐like cells in the overall pSS patient population (n = 25), pSS with only glandular symptoms (n = 10), pSS with extraglandular manifestations (EGMs) (n = 15), total SLE patient group (n = 25), SLE with SLE Disease Activity Index (SLEDAI) < 6 (n = 17), SLE with SLEDAI > 6 (n = 8) and control subjects (n = 21). (b) Ratio of CD4+CXCR5+ICOS+PD‐1+ Tfh‐like cells according to the presence of autoantibodies; in anti‐Ro/SSA antibody‐negative group (n = 16), anti‐Ro/SSA antibody‐positive group (n = 9), anti‐dsDNA negative group (n = 10), anti‐dsDNA‐positive group (n = 15) and control subjects (n = 21). (c) Distribution of IL‐21‐producing CD4+CXCR5+ PD‐1+ Tfh‐like cells. (d) Percentages of IL‐21‐producing CD4+CXCR5+PD‐1+ Tfh‐like cells according to the presence of autoantibodies in patient groups. Each data point represents an individual subject; horizontal lines show the mean values with standard deviation (s.d.). Statistically significant differences are indicated by *P < 0·05; **P < 0·01; ***P < 0·001.
We also determined CD45RA+ naive CD4+ T cells and CD45RA− activated or memory CD4+ T cells in a smaller groups of patients and controls (pSS n = 9, SLE n = 8 and control n = 6). We then measured the ratio of CXCR5+ICOS+ or CXCR5+PD‐1+ cells in both CD4+CD45RA− and CD4+CD45RA+ subsets. Naive CD45RA+ T cells showed mainly reduced ICOS and PD‐1 expression compared to activated or memory CD45RA− T cells. The ratios of CXCR5+ICOS+ cells were increased in patients with pSS and SLE in the CD4+CD45RA+ subset, although the values were generally under 0·25%. The percentages of CXCR5+ICOS+ cells were especially elevated in SLE, and the values varied between 0·120 and 1·310%. The ratios of CXCR5+PD‐1+ cells were also increased in both diseases and showed a similar tendency: the values were generally less than 0·68% in the CD4+CD45RA+ subset, while those in the CD4+CD45RA− T cells were between 1·280 and 12·10%. Moreover, we investigated the association between the ratios of CXCR5+ICOS+ cells and CXCR5+PD‐1+ cells in the CD4+CD45RA− subsets, and we found a significant positive correlation in the whole measured group (R = 0·6393, respectively, P = 0·0010) (Fig. S2).
Measurement of individual cytokine production of circulating Tfh cells
To establish the cytokine profile of circulating Tfh‐like cells, we determined intracellular IL‐21 cytokine production. The frequency of IL‐21‐producing CD4+CXCR5+PD‐1+ cells did not differ significantly between the whole group of pSS patients and healthy controls. However, we found that the percentages of CD4+CXCR5+PD‐1+IL‐21+ T cells were significantly higher in pSS patients with EGMs than control values (0·2449 ± 0·1657% versus 0·1469 ± 0·0649%, respectively, P = 0·0442) (Fig. 2c). The ratio of IL‐21‐producing Tfh‐like cells was elevated significantly in the overall SLE group, and in both SLE subgroups (SLEDAI < 6 and SLEDAI > 6 groups) compared to those measured in controls (0·3125 ± 0·1886% versus 0·1469 ± 0·0649%, respectively, P = 0·0003, 0·2786 ± 0·1805% versus 0·1469 ± 0·0649%, respectively, P = 0·0100 and 0·3846 ± 0·1968% versus 0·1469 ± 0·0649%, respectively, P = 0·0123) (Fig. 2c). When we measured the percentages of Tfh cells in patients with pSS with an emphasis on the presence of autoantibodies, the percentages of Tfh cells showed a significant 1·8‐fold increase in the anti‐Ro/SSA antibody‐positive pSS group compared to controls (0·2742 ± 0·1418% versus 0·1469 ± 0·0649%, respectively, P = 0·0297) (Fig. 2d). In SLE patients, we found significant 2.5‐fold higher percentages of Tfh cells in the anti‐dsDNA antibody‐positive SLE group compared to healthy controls (0·3638 ± 0·1982% versus 0·1469 ± 0·0649%, respectively, P = 0·0009) and 1·5‐fold higher ratios of Tfh cells compared to the anti‐dsDNA antibody‐negative SLE group (0·3638 ± 0·1982% versus 0·2356 ± 0·1509%, respectively, P = 0·0489) (Fig. 2d). Additionally, we investigated the possible associations between the ratio of CD4+CXCR5+ICOS+PD‐1+ Tfh‐like cells and IL‐21‐producing CD4+CXCR5+PD‐1+ T cells. A significant positive correlation was found in the pSS patient group (R = 0·5915, respectively, P = 0·0018) and a positive but not significant association observed in the SLE patient group (R = 0·3862, respectively, P = 0·0566) (Fig. 3).
Figure 3.
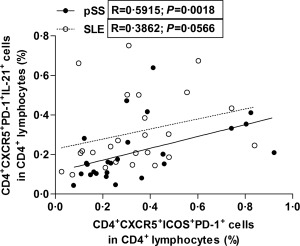
Circulating follicular T helper cells (Tfh)‐like cells are associated with the expansion of interleukin (IL)‐21‐producing Tfh‐like cells. Correlation between the percentages of CD4+CXCR5+ICOS+programmed death (PD)‐1+ Tfh‐like cells and CD4+CXCR5+PD‐1+IL‐21+ Tfh‐like cells in patients with primary Sjögren's syndrome (pSS) (black dots with solid line) and systemic lupus erythematosus (SLE) (clear dots with dashed line).
Assessment of IL‐10‐producing B cells
As a next step, we cultured PBMCs for 5 or 48 h, stimulated them with PIB alone or in combination with CpG, and then determined the ratios of IL‐10‐producing CD19+ B (B10) cells. After 5 h incubation with PIB+CpG, we found no significant differences in the ratio of IL‐10+CD19+ B cells between patient groups and healthy controls (Fig. 4a). However, after a 48‐h incubation period and stimulation with PIB alone for the last 5 h, we found that the percentages of IL‐10+CD19+ B cells in the pSS patient group were decreased significantly compared to those measured in the controls and SLE patient groups (0·9688 ± 0·4284% versus 1·592 ± 0·8799%, respectively, P = 0·0076 and 0·9688 ± 0·4284% versus 1·645 ± 1·198%, respectively, P = 0·0127) (Fig. 4b). Furthermore, IL‐10+CD19+ B cell percentages were determined after in‐vitro B10 progenitor (B10PRO) cell maturation by stimulation with CpG for 48 h with PIB added to the culture for the final 5 h. The total frequency of IL‐10‐producing CD19+ B cells, including B10 and matured B10PRO cells, was elevated significantly compared with PIB alone‐treated cells in each equivalent group (control: 5·543 ± 2·372% versus 1·592 ± 0·8799%, respectively, P < 0·0001; pSS: 5·712 ± 0·3·310% versus 0·9688 ± 0·4284%, respectively, P < 0·0001 and SLE: 6·342 ± 4·023% versus 1·645 ± 1·198%, respectively, P < 0·0001) (Fig. 4b). However, there were no significant differences between the patient groups and healthy controls regarding the distribution of IL‐10+CD19+ B cells after 48 h of CpG stimulation.
Figure 4.
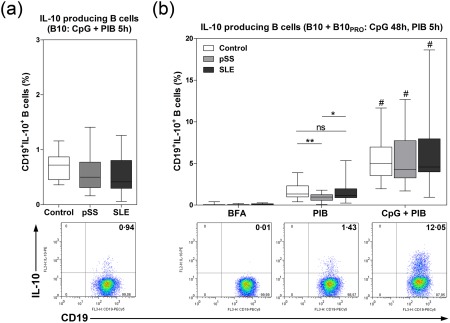
Quantification of peripheral interleukin (IL)‐10‐producing B10 and B10PRO cells in primary Sjögren's syndrome (pSS) patients, systemic lupus erythematosus (SLE) patients and healthy controls. Peripheral blood mononuclear cells (PBMCs) were isolated from 25 pSS patients, 25 SLE patients and 21 healthy controls; to gain B10 cell frequencies, cells were then incubated with cytosine–phosphate–guanosine (CpG) + phorbol 12‐myristate 13‐acetate (PMA) + ionomycin + brefeldin A (PIB) for 5 h. B10 + B10PRO cell maturation was induced with CpG stimulation for 48 h with PIB added during the last 5 h. Regarding the exact assessment of IL‐10 production, we used brefeldin A (BFA) and PIB alone controls during the procedure. Representative dot‐plots show IL‐10‐producing B cells in response to different conditions. (a) Percentages of IL‐10+CD19+ B10 cells. (b) Percentages of IL‐10+CD19+ B10 and B10PRO cells in the pSS patient group (n = 25), SLE patient group (n = 25) and control group (n = 21) in response to BFA, PIB or CpG + PIB. Results are presented as a box‐and‐whiskers plot, horizontal lines show the mean values. Statistically significant differences are indicated by *P < 0·05; **P < 0·01; n.s. = no significant differences between patient and control groups within an experiment. Statistically significant differences are indicated by #P < 0·0001 between PIB alone and PIB + CpG stimulation within the groups.
Correlation analysis between peripheral Tfh‐like cells and certain B cell subpopulations in pSS
We measured the relationship between the percentages of CD19+CD38hiCD24hiCD27− transitional B cells and the proportions of both CD4+CXCR5+ICOS+PD‐1+ Tfh‐like cells and CD4+CXCR5+PD‐1+IL‐21+ Tfh cells in pSS. We revealed a positive but not significant correlation between the ratio of transitional and Tfh‐like cells (R = 0·3389, respectively, P = 0·0975; moreover, a significant positive correlation between transitional B cells and IL‐21‐producing Tfh cells in the overall pSS group (R = 0·4455, respectively, P = 0·0256) (data not shown). When we focused only on pSS patients with EGMs, we revealed strong significant positive correlations between the aforementioned cell subsets (transitional B versus Tfh‐like cells: R = 0·5964, respectively, P = 0·0189 and transitional B versus IL‐21+ Tfh‐like cells R = 0·5857, respectively, P = 0·0218) (Fig. 5a,b). Similar observations were found between the ratios of CD19+CD38+CD24+ mature‐naive B and CD4+CXCR5+PD‐1+IL‐21+ Tfh cells in pSS. A significant positive correlation was found in the whole pSS group (R = 0·5058, respectively, P = 0·0095; data not shown) and in patients with EGMs; nevertheless, the association was more powerful in the latter case (R = 0·5536, respectively, P = 0·0323) (Fig. 5c).
Figure 5.
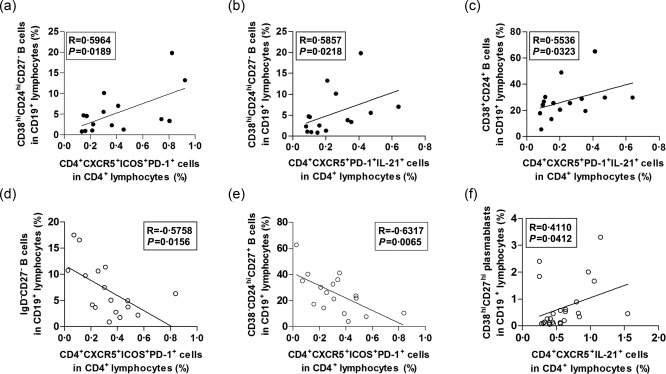
Correlation analysis between peripheral follicular T helper cells (Tfh)‐like cells and transitional B cell subpopulations. (a) Correlation between the percentages of Tfh‐like cells and CD19+CD38hiCD24hiCD27− transitional B cells in primary Sjögren's syndrome (pSS) patients with extraglandular manifestations (EGMs). (b) Correlation between the percentages of interleukin (IL)‐21‐producing Tfh‐like cells and transitional B cells in pSS patients with EGMs. (c) Correlation between the proportions of IL‐21‐producing Tfh‐like cells and CD19+CD38+CD24+ mature‐naive B cells in pSS patients with EGMs. (d) Correlation between the ratios of Tfh‐like cells and CD19+IgD−CD27− double negative (DN) B cells in SLE patients with SLE Disease Activity Index (SLEDAI) < 6. (e) Correlation between the frequencies of Tfh‐like cells and CD19+CD38−CD24hiCD27+ primarily memory B cells in SLE patients with SLEDAI < 6. (f) Correlation between the percentages of IL‐21‐producing CD4+CXCR5+ T cells and CD19+CD38hiCD27hi plasmablasts in the whole SLE patient group. Each data point represents an individual subject.
Correlation analysis between peripheral Tfh‐like cells and certain B cell subsets in SLE
We measured the association between CD4+CXCR5+ICOS+PD‐1+ Tfh‐like cells and both CD19+IgD−CD27− DN B cells and CD19+CD38−CD24hiCD27+ primarily memory B cells in SLE. A significant negative correlation was observed between the percentages of DN B cells and Tfh‐like cells in SLE patients with SLEDAI < 6 (R = −0·5758, respectively, P = 0·0156) (Fig. 5d). Furthermore, a significant negative correlation was also found between the ratios of primarily memory B cells and Tfh‐like cells in the same subgroup (R = −0·6317, respectively, P = 0·0065) (Fig. 5e). Of note, these differences were also observed tendentiously in the whole SLE group; however, the correlations were not significant (data not shown). Conversely, a significant positive correlation was revealed between the proportions of CD4+CXC5+IL‐21+ T cells and CD19+CD38hiCD27hi plasmablasts in the whole SLE patient group (R = 0·4110, respectively, P = 0·0412) (Fig. 5f).
Association of peripheral Tfh‐like cells, B cell subsets with serological parameters
We examined the possible relationship between circulating Tfh‐like cells or IL‐21‐producing Tfh‐like cells and serological markers (Table 1) and between certain B cell subsets and serological markers (Table 2).
Table 1.
Association of certain follicular T helper cells (Tfh)‐like cell proportions with serological parameters.
| Association | Disease | Variable | Correlation coefficient | P‐value |
|---|---|---|---|---|
| Positive | pSS | CD4+CXCR5+ICOS+PD‐1+ Tfh‐like cells versus levels of serum IgG antibody |
Spearman's R = 0·6143 |
0·0018 |
| CD4+CXCR5+ICOS+PD‐1+ Tfh‐like cells versus levels of serum RF |
Spearman's R = 0·6219 |
0·0101 | ||
| CD4+CXCR5+PD‐1+IL‐21+ Tfh cells versus levels of serum IC |
Pearson's R = 0·5734 |
0·0128 | ||
| SLE | CD4+CXCR5+IL‐21+ Tfh cells versus levels of serum IC |
Spearman's R = 0·4723 |
0·0478 |
IC = immune complex; ICOS = inducible T cell co‐stimulator; Ig = immunoglobulin; PD‐1 = programmed death 1; pSS = primary Sjögren's syndrome; RF = rheumatoid factor; SLE = systemic lupus erythematosus.
Table 2.
Association of certain B cell proportions with laboratory parameters.
| Association | Disease | Variable | Correlation coefficient | P‐value |
|---|---|---|---|---|
| Positive | pSS | CD19+CD38hiCD24hiCD27− transitional B cells versus levels of serum IgG |
Spearman's R = 0·4884 |
0·0181 |
| CD19+CD38hiCD27hi plasmablasts versus levels of serum IC |
Spearman's R = 0·4907 |
0·0387 | ||
| SLE | CD19+CD38hiCD27hi plasmablasts versus levels of serum anti‐dsDNA |
Spearman's R = 0·5320 |
0·0090 | |
| Negative | SLE | CD19+CD38hiCD24hiCD27− transitional B cells versus levels of serum C3 |
Pearson's R = −0·4914 |
0·0172 |
IC = immune complex; Ig = immunoglobulin; pSS = primary Sjögren's syndrome; SLE = systemic lupus erythematosus.
Discussion
Patients with pSS and SLE are characterized by fundamental disturbances in the proportion of different B cell subpopulations, both in the peripheral blood and at the site of inflammation. In our study, we found a significant enrichment of CD19+IgD+CD27− naive B cells in the peripheral blood of both pSS and SLE patients compared to healthy individuals. This observation is consistent with previous reports 12, 13, 14 and indicates that early B cell tolerance checkpoints are impaired significantly in these autoimmune diseases; moreover, the break of tolerogenic mechanism at this stage probably accelerates the mobilization of autoreactive naive B cells from the bone marrow to the periphery 15, 16.
There is another major tolerance checkpoint during the maturation stage of immature B cells when transitional B cells overcome a negative selection. In healthy adults, only a small portion of peripheral B cells are CD19+CD38hiCD24hiCD27− transitional B cells, and most of them belong to the mature‐naive and memory B cell pool. The pathological accumulation of these cells may occur due to their increased exiting from the bone marrow or disturbed entrance into secondary lymphoid organs 17. In accordance with previous findings 17, 18, 19, we observed significant elevation in the percentages of transitional B cells in SLE patients; additionally, this cell population showed association with the disease activity. In pSS, the frequency of transitional B cells did not correlate with the presence of EGMs. However, when we divided pSS patients into subgroups based on the presence of anti‐SSA/Ro autoantibodies, we observed significantly higher transitional B cell proportions in pSS patients with autoantibody positivity, and found a positive association between elevated cell ratios and serum IgG levels.
When the transitional B cells undergo maturation processes, mature‐naive B cells are generated which circulate into B cell follicles in secondary lymphoid organs 20. Of note, the defect in early self‐tolerance may also cause the expansion of circulating self‐reactive and polyreactive type of mature‐naive B cell subset. In our study, we measured significantly higher percentages of CD19+CD38+CD24+ mature‐naive B cells in SLE. Importantly, large numbers of autoreactive B cells occur among the mature‐naive B cell compartment in SLE 21.
We also confirmed that peripheral CD19+IgD+CD27+ non‐switched memory B cells and CD19+IgD−CD27+ switched memory B cells are diminished strongly in both pSS and SLE 17, 22, 23, 24. Additionally, we revealed significant differences between the distributions of the two memory B cell compartments in the investigated diseases. In pSS patients, the proportion of switched memory B cells decreased significantly, while in SLE patients the non‐switched memory B cells reduced significantly. Furthermore, within both the pSS and SLE patient groups, a more pronounced reduction was observed in patients with EGMs or higher SLEDAI values. In addition, among SLE patients, individuals with active disease status exhibited a significant decrease in the switched memory B cell subset, which underlines the importance of the changing distribution of B cell subsets during the disease course. The lower ratio of circulating memory B cells may be explained by the over‐expression of chemokine molecules CXCR3 and CXCR4 which guide them into the inflamed tissues 12, 13, 25. Recent findings indicate that CD19+IgD+CD27+ non‐switched memory B cells from SLE patients are in an activated state and exhibit elevated levels of activation‐induced cytidine deaminase (AID), which promotes their differentiation into IgG‐secreting plasma cells 12. The lower ratio of CD19+IgD−CD27+ switched memory B cells in pSS can also be explained by the pronounced differentiation towards plasma cells or by the shedding of CD27 from the surface of memory B cells 26. We also identified CD19+CD38−CD24hiCD27+ primarily memory B cells which, similarly to non‐switched memory B cells, showed a significant elevation in SLE patients with active disease status.
We detected significantly increased CD19+IgD−CD27− DN B cells in SLE patients, while the ratio of DN B cells was decreased significantly in pSS patients. However, regarding the clinical features, these differences were found only in patients with a more pronounced disease course, such as high SLEDAI or the presence of EGMs. A previous report proposed that the presence of these cells could be the result of an extrafollicular differentiation process in secondary lymphoid organs. The expression of CD95 indicates that they are in an activated state; however, their activation does not require T cell interaction. Furthermore, due to the expression of CXCR3, after receiving activation signals, DN B cells could migrate to inflamed tissues 27, 28. The proportion of CD19+CD38hiCD27hi plasmablasts was decreased significantly in pSS patients with EGMs, while their ratio was elevated significantly in SLE patients with SLEDAI > 6. This discrepancy may arise from the heightened expression of CXCR3 and CXCR4 on plasma cells and plasmablasts of patients with pSS, which leads to their migration towards the site of inflammation or even the bone marrow 29, 30, 31. Moreover, in SLE, the differentiation towards plasmablasts is more pronounced compared to pSS, and could be originated from both T‐dependent and T‐independent responses.
Tfh cells are crucial immune regulators in secondary lymphoid follicles by controlling B cell proliferation and differentiation. As the measurement of human GC Tfh cell from lymphoid follicles could hardly be performed, the investigation of circulating Tfh‐like cells are widely accepted. In recent years a number of observations have indicated that circulating Tfh cells represent a partial differentiated state; however, they express Tfh‐related markers at lower intensity and maintain functional characteristics to promote B cell differentiation 32, 33. At the periphery, Tfh‐like cells may represent a memory CD4+ T cell condition, and according to certain cell surface molecules, including ICOS, PD‐1 and CCR7, they can be subdivided into distinct subsets 34. In the present study, we revealed a significant increase in the proportion of peripheral CD4+CXCR5+ICOS+PD‐1+ Tfh‐like cells in pSS patients with EGMs compared to control values, while values of pSS patients without EGMs were similar to healthy individuals. Moreover, we also observed a significant difference between patients with and without EGMs. With this independent set of pSS cases, we reinforced our previous findings 6. When we divided patients into subgroups based on the presence of anti‐SSA/Ro, we found significantly higher Tfh‐like cell percentages in autoantibody‐positive group. The same changes were measured in SLE, and circulating Tfh‐like cell percentages were elevated significantly in patients positive for anti‐dsDNA; however, there was no significant difference between the inactive and active status of the disease.
As a next step, we evaluated the proportion of IL‐21‐producing CD4+CXCR5+PD‐1+ Tfh‐like cells and found a significant elevation in pSS patients with EGMs and/or anti‐SSA/Ro positivity. Similarly, a marked expansion was observed in SLE patients which was independent of the disease status; however, the tendency was more pronounced in patients with anti‐dsDNA autoantibody. Previous investigations on Tfh‐like cells led to similar results 35, 36, 37, 38, 39, 40, 41, although the identification of these cell types are not yet characterized fully. For that reason, some differences could arise with regard to the number of cell surface markers or the exact percentages of Tfh‐like cells; nevertheless, there is broad agreement in that the expansion of Tfh‐like cells in peripheral blood could reflect the severity of autoimmune disorders.
To gain a better view on the significance of Tfh‐like cell expansion in pSS and SLE, we analysed the association between the proportion of Tfh‐like like cells and other parameters. Our results revealed a positive correlation between circulating Tfh‐like cells and serum levels of IgG and RF. Additionally, IL‐21‐producing Tfh‐like cells showed a positive association with serum levels of circulating ICs in both pSS and SLE patients. These parameters are related strongly to the regulation of plasma cell generation which underlines the role of Tfh cells in autoimmune diseases. Regarding B cell subsets, the frequency of transitional B cells correlated with the increased Tfh‐like cells and IL‐21+ Tfh‐like cell percentages in pSS; furthermore, the level of mature‐naive B cells also correlated with the increased ratio of Tfh‐like cells. These observations suggest that disturbances in early B cell tolerance mechanisms may lead to the peripheral expansion and further accumulation of these B cell subsets. The mutual co‐operation between B cells and Tfh cells contributes potentially to the development of characteristic pSS features. Conversely, the expansion of circulating Tfh‐like cells correlated negatively with both primarily memory B cells and DN B cells in SLE patients, which indicates the role of Tfh cells in B cell differentiation skewing towards plasma cell direction. By demonstrating a positive association between IL‐21‐producing CD4+CXCR5+ T cells and plasmablasts, we underlined the importance of IL‐21 in the pathogenesis of SLE.
In the present study, we analysed the whole population of IL‐10‐producing B cells. The decreased IL‐10‐producing Breg cell percentages in pSS may elucidate our previous observations that, despite the increased peripheral IL‐10‐producing regulatory T (Tr1) cell proportions, a significant decrease in soluble IL‐10 levels can be detected in the disease. We assume that, along with the decrease in IL‐10 production of Breg cells, the intensification of a counterbalance mechanism appears; thus, levels of IL‐10‐producing Tr1 cells increase as a feedback process attempting to compensate the progression of disproportional immune responses 42.
In conclusion, our observations underline the essential role of the profound expansion of circulating Tfh‐like cells and their IL‐21 production in combination with the characteristic abnormal distribution of peripheral B cell subsets disturbances in the pathogenesis of both pSS and SLE (Fig. 6). We believe that these extensive experiments reveal the importance of Tfh cells in these autoimmune diseases and provide valuable knowledge to invent novel therapeutic strategies targeting certain B cell subsets and suppressing Tfh‐mediated responses.
Figure 6.
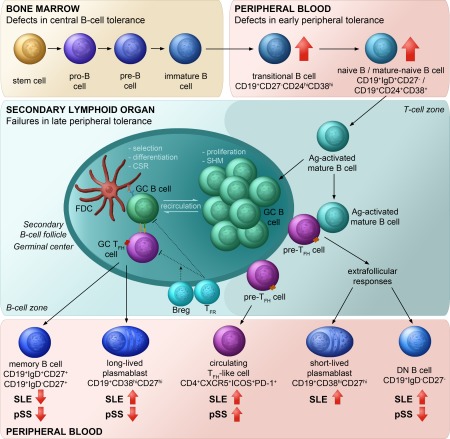
Summarized model showing the outcome of disturbed tolerance during B cell development in primary Sjögren's syndrome (pSS) and systemic lupus erythematosus (SLE). In physiological circumstances, to avoid autoreactivity, during B cell development multiple tolerance checkpoints are applied for the selection of autoreactive B cells 43. Abnormalities in checkpoint regulation, along with hyperactivation of Tfh cells and self‐reactive B cells contribute to the development of autoimmunity in susceptible individuals. Breaking of early B cell tolerance leads to consequential accumulation of transitional and naive B cells in the periphery. Perturbation of late peripheral tolerance in secondary lymphoid organs allows autoreactive mature B cells to undergo extrafollicular or germinal centre responses, which are supported by Tfh cells, and differentiate into memory B cells or plasmablasts 15, 44, 45. Derailed B cell homeostasis and increased Tfh cells responses manifest in characteristic changes in B cell distribution and accumulation of circulating Tfh‐like cells in pSS and SLE.
Disclosure
The authors declare that they have no disclosures.
Author contributions
K.Sz. and G.P. participated in the study design, performed laboratory experiments, collected, statistically analysed and interpreted the data, and drafted the manuscript. A.Sz. and T.T. participated in the interpretation of clinical data. M.Z. designed the study, interpreted the data and approved the final version of the manuscript for publication. All authors read and approved the final manuscript.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Viability of the cytosine–phosphate–guanosine (CpG)‐exposed cells were determined by 7‐aminoactinomycin‐D (7‐AAD) staining using flow cytometric analysis. (a) Representative dot‐plots show the 7‐AAD+ dead cells and 7‐AAD− viable cells in the peripheral blood mononuclear cells (PBMCs). Underneath, smaller dot‐plots indicate the differences in the presence of B cells among the viable or dead cell populations. (b) Representative dot‐plots show the frequency of CD19+7‐AAD+ cells within the lymphocytes. (c) Percentages of 7‐AAD+ (left) and CD19+7‐AAD+ cells (right) in the primary Sjögren's syndrome (pSS) patient group (n = 5), systemic lupus erythematosus (SLE) patient group (n = 7) and control group (n = 4) in response to brefeldin A (BFA), phorbol 12‐myristate 13‐acetate (PMA) + ionomycin + brefeldin A (PIB) or CpG + PIB.
Fig. S2. Evaluation of follicular T helper cells (Tfh) markers on naive CD45RA+ T cells and active or memory CD45RA− T cells in primary Sjögren's syndrome (pSS) patients, systemic lulus erythematosus (SLE) patients and healthy subjects. Peripheral blood mononuclear cells (PBMCs) were isolated from nine pSS patients, eight SLE patients and six healthy controls were then stained as described previously. Representative dot‐plots show the gating strategy for the determination of CD4+CD45RA− and CD4+CD45RA+ subsets. (a) Percentages of CXCR5+ inducible T cell co‐stimulator (ICOS)+ in the CD4+CD45RA− (left) and in the CD4+CD45RA+ (right) subsets. (b) Percentages of CXCR5+programmed death 1 (PD)‐1+ in the CD4+CD45RA− (left) and in the CD4+CD45RA+ (right) subsets. (c) Association between the ratio of CXCR5+ICOS+ cells and CXCR5+PD‐1+ cells in the CD4+CD45RA− subset. Each data point represents an individual subject; horizontal lines show the mean values with standard deviation (s.d.).
Acknowledgements
Experimental work was performed with the support of the Hungarian National Scientific Research Fund (OTKA Grant no. K101470) and the TAMOP 4.2.2.A‐11/1/KONV‐2012‐0023 ‘DEFENSE‐NET’ project. The project is co‐financed by the European Union and the European Social Fund.
References
- 1. Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol 2013; 10:122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papp G, Szabó K, Szekanecz Z, Zeher M. Follicular helper T cells in autoimmune diseases. Rheumatology (Oxf) 2014; 53:1159–60. [DOI] [PubMed] [Google Scholar]
- 3. Simpson N, Gatenby P, Wilson A et al Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum 2010; 62:234–44. [DOI] [PubMed] [Google Scholar]
- 4. Feng X, Wang D, Chen J et al Inhibition of aberrant circulating Tfh cell proportions by corticosteroids in patients with systemic lupus erythematosus. PLOS ONE 2012; 7:e51982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeher M. Sjögren's syndrome In: Zeher M, Szodoray P, eds. Sjögren's syndrome and associated disorders. Kerala, India: Transworld Research Network, 2009:1–25. [Google Scholar]
- 6. Szabo K, Papp G, Barath S, Gyimesi E, Szanto A, Zeher M. Follicular helper T cells may play an important role in the severity of primary Sjögren's syndrome. Clin Immunol 2013; 147:95–104. [DOI] [PubMed] [Google Scholar]
- 7. Maehara T, Moriyama M, Hayashida J et al Selective localization of T helper subsets in labial salivary glands from primary Sjögren's syndrome patients. Clin Exp Immunol 2012; 169:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Szabo K, Papp G, Dezso B, Zeher M. The histopathology of labial salivary glands in primary Sjögren's syndrome: focusing on follicular helper T cells in the inflammatory infiltrates. Mediat Inflamm 2014; 2014:631787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vitali C, Bombardieri S, Jonsson R et al Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis 2002; 61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 11. Petri M, Orbai AM, Alarcón GS et al Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodríguez‐Bayona B, Ramos‐Amaya A, Pérez‐Venegas JJ, Rodríguez C, Brieva JA. Decreased frequency and activated phenotype of blood CD27 IgD IgM B lymphocytes is a permanent abnormality in systemic lupus erythematosus patients. Arthritis Res Ther 2010; 12:R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hansen A, Odendahl M, Reiter K et al Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjögren's syndrome. Arthritis Rheum 2002; 46:2160–71. [DOI] [PubMed] [Google Scholar]
- 14. Bohnhorst J, Bjørgan MB, Thoen JE, Natvig JB, Thompson KM. Bm1‐Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjögren's syndrome. J Immunol 2001; 167:3610–8. [DOI] [PubMed] [Google Scholar]
- 15. Corsiero E, Sutcliffe N, Pitzalis C, Bombardieri M. Accumulation of self‐reactive naïve and memory B cell reveals sequential defects in B cell tolerance checkpoints in Sjögren's syndrome. PLOS ONE 2014; 9:e114575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mietzner B, Tsuiji M, Scheid J et al Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci USA 2008; 105:9727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wehr C, Eibel H, Masilamani M et al A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol 2004; 113:161–71. [DOI] [PubMed] [Google Scholar]
- 18. Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood 2005; 105:4390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blair PA, Noreña LY, Flores‐Borja F et al CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010; 32:129–40. [DOI] [PubMed] [Google Scholar]
- 20. Dörner T, Jacobi AM, Lee J, Lipsky PE. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J Immunol Methods 2011; 363:187–97. [DOI] [PubMed] [Google Scholar]
- 21. Yurasov S, Wardemann H, Hammersen J et al Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med 2005; 201:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Odendahl M, Jacobi AM, Hansen A et al Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol 2000; 165:5970–9. [DOI] [PubMed] [Google Scholar]
- 23. Bohnhorst J, Thoen J, Natvig J, Thompson K. Significantly depressed percentage of CD27+ (memory) B cells among peripheral blood B cells in patients with primary Sjögren's syndrome. Scand J Immunol 2001; 54:421–7. [DOI] [PubMed] [Google Scholar]
- 24. Korganow AS, Knapp AM, Nehme‐Schuster H et al Peripheral B cell abnormalities in patients with systemic lupus erythematosus in quiescent phase: decreased memory B cells and membrane CD19 expression. J Autoimmun 2010; 34:426–34. [DOI] [PubMed] [Google Scholar]
- 25. Hansen A, Reiter K, Ziprian T et al Dysregulation of chemokine receptor expression and function by B cells of patients with primary Sjögren's syndrome. Arthritis Rheum 2005; 52:2109–19. [DOI] [PubMed] [Google Scholar]
- 26. Bohnhorst J, Bjørgan M, Thoen J, Jonsson R, Natvig J, Thompson K. Abnormal B cell differentiation in primary Sjögren's syndrome results in a depressed percentage of circulating memory B cells and elevated levels of soluble CD27 that correlate with serum IgG concentration. Clin Immunol 2002; 103:79–88. [DOI] [PubMed] [Google Scholar]
- 27. Jacobi AM, Reiter K, Mackay M et al Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis Rheum 2008; 58:1762–73. [DOI] [PubMed] [Google Scholar]
- 28. Wei C, Anolik J, Cappione A et al A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol 2007; 178:6624–33. [DOI] [PubMed] [Google Scholar]
- 29. Szyszko EA, Brun JG, Skarstein K, Peck AB, Jonsson R, Brokstad KA. Phenotypic diversity of peripheral blood plasma cells in primary Sjögren's syndrome. Scand J Immunol 2011; 73:18–28. [DOI] [PubMed] [Google Scholar]
- 30. Harada Y, Kawano MM, Huang N et al Identification of early plasma cells in peripheral blood and their clinical significance. Br J Haematol 1996; 92:184–91. [DOI] [PubMed] [Google Scholar]
- 31. Jacobi AM, Odendahl M, Reiter K et al Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum 2003; 48:1332–42. [DOI] [PubMed] [Google Scholar]
- 32. Morita R, Schmitt N, Bentebibel S et al Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsai LM, Yu D. Follicular helper T‐cell memory: establishing new frontiers during antibody response. Immunol Cell Biol 2014; 92:57–63. [DOI] [PubMed] [Google Scholar]
- 34. He J, Tsai LM, Leong YA et al Circulating precursor CCR7(lo)PD‐1(hi) CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 2013; 39:770–81. [DOI] [PubMed] [Google Scholar]
- 35. Terrier B, Costedoat‐Chalumeau N, Garrido M et al Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. J Rheumatol 2012; 39:1819–28. [DOI] [PubMed] [Google Scholar]
- 36. Xu H, Liu J, Cui X et al Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L‐dependent manner. Cell Immunol 2015; 295:46–51. [DOI] [PubMed] [Google Scholar]
- 37. Zhang X, Lindwall E, Gauthier C et al Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus 2015; 24:909–17. [DOI] [PubMed] [Google Scholar]
- 38. Li X, Wu Z, Ding J et al Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjögren's syndrome. Biochem Biophys Res Commun 2012; 422:238–44. [DOI] [PubMed] [Google Scholar]
- 39. Wang L, Zhao P, Ma L et al Increased interleukin 21 and follicular helper T‐like cells and reduced interleukin 10+ B cells in patients with new‐onset systemic lupus erythematosus. J Rheumatol 2014; 41:1781–92. [DOI] [PubMed] [Google Scholar]
- 40. Choi JY, Ho JH, Pasoto SG et al Circulating follicular helper‐like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol 2015; 67:988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le Coz C, Joublin A, Pasquali JL, Korganow AS, Dumortier H, Monneaux F. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLOS ONE 2013; 8:e75319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Szodoray P, Papp G, Horvath I et al Cells with regulatory function of the innate and adaptive immune system in primary Sjögren's syndrome. Clin Exp Immunol 2009; 157:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Browning JL. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat Rev Drug Discov 2006; 5:564–76. [DOI] [PubMed] [Google Scholar]
- 44. Guerrier T, Youinou P, Pers JO, Jamin C. TLR9 drives the development of transitional B cells towards the marginal zone pathway and promotes autoimmunity. J Autoimmun 2012; 39:173–9. [DOI] [PubMed] [Google Scholar]
- 45. Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity 2007; 26:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Viability of the cytosine–phosphate–guanosine (CpG)‐exposed cells were determined by 7‐aminoactinomycin‐D (7‐AAD) staining using flow cytometric analysis. (a) Representative dot‐plots show the 7‐AAD+ dead cells and 7‐AAD− viable cells in the peripheral blood mononuclear cells (PBMCs). Underneath, smaller dot‐plots indicate the differences in the presence of B cells among the viable or dead cell populations. (b) Representative dot‐plots show the frequency of CD19+7‐AAD+ cells within the lymphocytes. (c) Percentages of 7‐AAD+ (left) and CD19+7‐AAD+ cells (right) in the primary Sjögren's syndrome (pSS) patient group (n = 5), systemic lupus erythematosus (SLE) patient group (n = 7) and control group (n = 4) in response to brefeldin A (BFA), phorbol 12‐myristate 13‐acetate (PMA) + ionomycin + brefeldin A (PIB) or CpG + PIB.
Fig. S2. Evaluation of follicular T helper cells (Tfh) markers on naive CD45RA+ T cells and active or memory CD45RA− T cells in primary Sjögren's syndrome (pSS) patients, systemic lulus erythematosus (SLE) patients and healthy subjects. Peripheral blood mononuclear cells (PBMCs) were isolated from nine pSS patients, eight SLE patients and six healthy controls were then stained as described previously. Representative dot‐plots show the gating strategy for the determination of CD4+CD45RA− and CD4+CD45RA+ subsets. (a) Percentages of CXCR5+ inducible T cell co‐stimulator (ICOS)+ in the CD4+CD45RA− (left) and in the CD4+CD45RA+ (right) subsets. (b) Percentages of CXCR5+programmed death 1 (PD)‐1+ in the CD4+CD45RA− (left) and in the CD4+CD45RA+ (right) subsets. (c) Association between the ratio of CXCR5+ICOS+ cells and CXCR5+PD‐1+ cells in the CD4+CD45RA− subset. Each data point represents an individual subject; horizontal lines show the mean values with standard deviation (s.d.).


