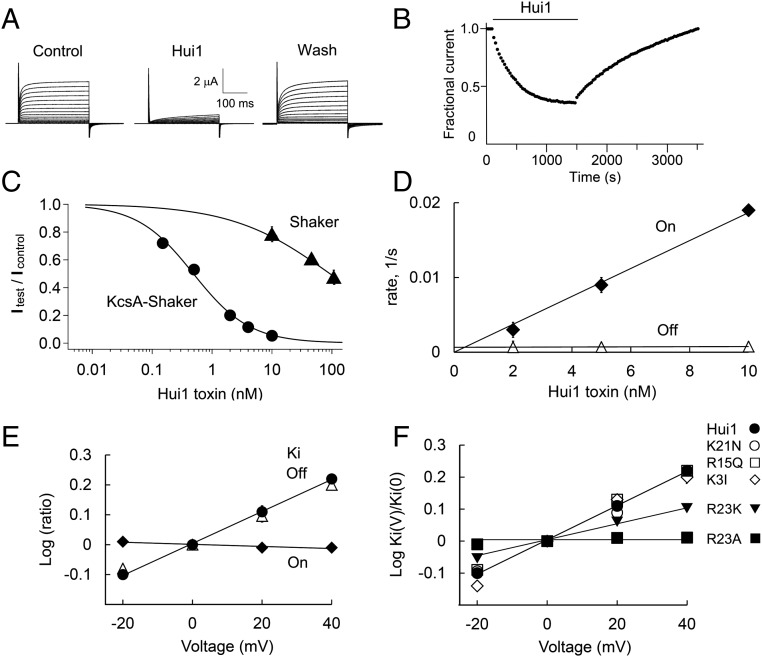
Fig. 3.
Hui1 is a potent, specific, and voltage-dependent blocker of KcsA. KcsA-Shaker and other ion channels as indicated were expressed in oocytes and studied by two-electrode voltage clamp to assess equilibrium inhibition and kinetic blocking parameters using a holding voltage of −80 mV, 300-ms test pulses, and a 10-s interpulse interval (Tables 1 and 2), n = 6–12 cells for each condition. Values are mean ± SEM. Some error bars are smaller than symbols. (A) Representative current traces for KcsA-Shaker channels at steady state before (control), in the presence of 2 nM Hui1 (Hui1), and after toxin washout (wash) with steps of 10 mV from −80 mV to 80 mV. (B) The time course for block and unblock of KcsA-Shaker on acute application (bar) and washout of 2 nM Hui1. Peak currents recorded at 0 mV; every third point is shown. (C) Dose–response relationships for Hui1 inhibition of KcsA-Shaker (●) and Shaker (▲) studied as in B and fit to the Hill relationship, Fun = (1 + ([Tx]/Ki)h)−1, where Fun is the fraction of unblocked current at equilibrium, Ki is the dissociation constant, h is the Hill coefficient, and [Tx] is the concentration of Hui1. The Ki of Hui1 for KcsA-Shaker channels was estimated from the fit to be 0.50 ± 0.03 nM with h = 0.93 ± 0.05. (D) Effect of Hui1 concentration on blocking kinetics of KcsA-Shaker. The apparent first-order rate constants for association (♦, On, kon[Tx]) and dissociation (△, Off, koff) are plotted as a function of Hui1 concentration using the protocol in B. (E) Effect of voltage on Hui1 blocking kinetics of KcsA-Shaker channels. Each parameter was measured with test steps from −20 mV to 40 mV and normalized to its value at 0 mV; ♦, kon; Δ, koff; ●, Ki. On-rate and off-rate time constants were determined by single-exponential fits to the time course for block or unblock on acute application or washout of 5 nM Hui1. The inhibition constant Ki was calculated from the fraction of unblocked current at equilibrium and the rate constants (Materials and Methods). (F) Effect of voltage on blockade of KcsA-Shaker by Hui1 mutants. Ki for each toxin was determined from −20 mV to 40 mV based on the fraction of unblocked current at equilibrium (Fun) and plotted as a ratio to the value at 0 mV. The change in Ki with voltage is due to altered off-rate.