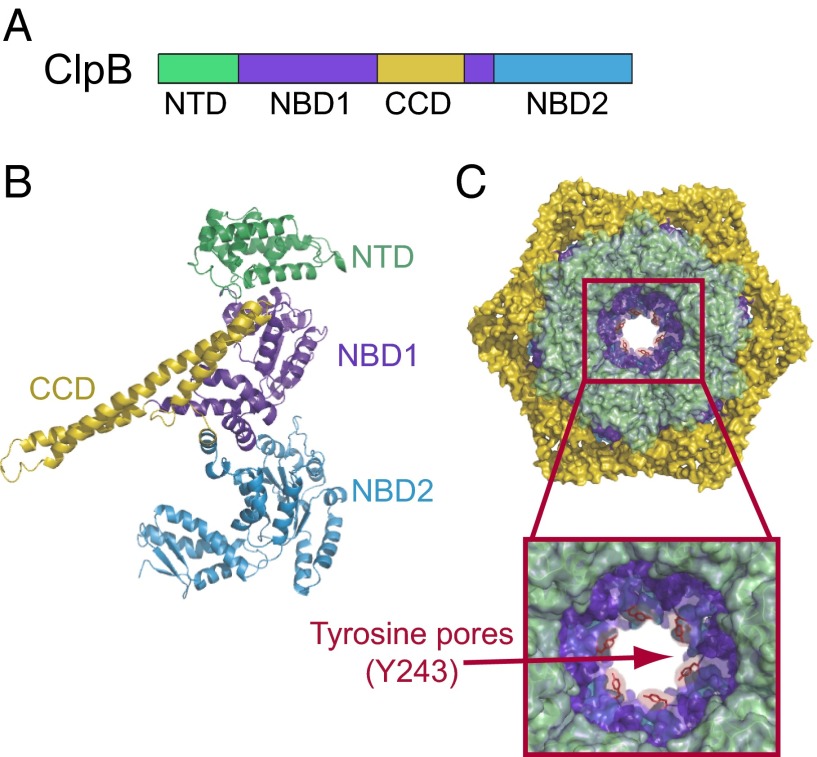
Fig. 1.
Structure and domain organization of the hexameric ClpB chaperone. Domain organization (A) and protomeric structure (B) of the ClpB chaperone [Protein Data Bank (PDB) ID code 1QVR (10)]. The ClpB protomer consists of an N-terminal domain (NTD; green), two nucleotide binding domains (NBD1, NBD2; dark and light blue, respectively), and a coil–coil domain insertion (CCD; yellow). (C) The monomers assemble into a hexamer consisting of three rings formed by NTDs (top ring; green), NBD1-CCD (blue-yellow), and NBD2 enclosing the central pore. The Inset shows a magnified view of the central pore loops of NBD1 with the conserved tyrosines (Y243; represented as red sticks) extending into the axial channel. This model of ClpB hexamers is based on cryo-electron microscopy structures of E. coli ClpB (EMD-2563) (52).