Figure EV1. Structural comparison of the conserved Bergerat fold in UL50 and UL53 with that of members of the GHKL superfamily and Bet3.
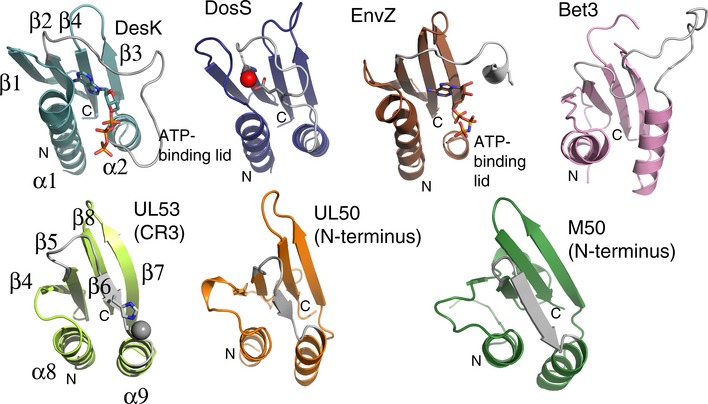
Ribbon representations of (from left to right in the top row) the ATP‐binding domains of three histidine kinase proteins (DesK, DosS, and EnvZ). The ATP molecules bound in DesK and EnV are shown in stick form, and the ATP‐binding lid is shown in gray. A short linker region (gray) on which a glutamate residue (in part) coordinates zinc (red sphere) takes the place of an ATP‐binding lid in DosS. The ATP‐binding lid is also missing in a portion of Bet3, a vesicle‐trafficking protein (top row, right), and the NEC subunits (bottom row, left to right) UL53 (comprising most of conserved region 3 (CR3)), and UL50 and M50, both comprising the N‐terminal portion of the proteins, where otherwise the Bergerat fold (α‐β‐β‐α‐β‐β topology) is fully conserved (the secondary elements of DesK and UL53 are labeled as an example of the fold sequence). The deviations observed between the structures at the ATP‐binding lid or equivalent region are shown in gray.
