Abstract
Back ground:
Atherosclerosis is the major cause of death. The most common risk factors are hyperlipidemia, diabetes, and other factors like chronic infection and inflammation.
Objective:
This study was undertaken to assess the effect of sitagliptin on atherosclerosis via interfering with inflammatory and oxidative pathways.
Materials and Methods:
A total of 18 local domestic male rabbits were included in this study. The animals were randomly divided into three groups (6 rabbits in each group): Group I normal were fed with chow (oxiod) diet for 12 weeks. Group II were fed with 1% cholesterol enriched diet for 12 weeks. Group III rabbits fed with cholesterol enriched diet for 6 weeks, and then continued on cholesterol enriched diet and treated with sitagliptin 125 mg/kg/day orally for the next 6 weeks. Blood samples were collected at the start of the study, at 6 weeks of the study and then at the end of treatment to measure serum lipids profile, hsCRP and TNFα. At end of the study, the aorta was removed for measurement of MDA, glutathione and, aortic intima-media thickness.
Results:
Sitagliptin results in a significant reduction (p < 0.05) in serum level of total cholesterol (TC), triglycerides (TG), high sensitive C-reactive protein (hsCRP) and TNFα with a significant increase (p < 0.05) in serum HDL level. There was a significant reduction (p < 0.05) in aortic MDA, in comparison to the untreated control group. Furthermore, sitagliptin causes significant increment (p < 0.05) in aortic GSH in comparison to induced untreated group. Regarding histopathological results, sitagliptin results in a significant reduction (p < 0.05) in atherosclerotic lesions in comparison to the induced untreated group and significant reduction in aortic intima-media thickness (p < 0.05).
Conclusion:
Sitagliptin reduced atherosclerosis progression in hyperlipidemic rabbit via its effect on lipid parameters and interfering with inflammatory and oxidative stress.
Keywords: Sitagliptin, atherosclerosis, oxidative stress, inflammation
Introduction
The endothelium has autocrine and paracrine components that regulate anti-inflammatory, mitogenic, and contractile activities of the vessel wall as well as the homeostatic process within the vessel lumen. Atherosclerosis is likely initiated when endothelial cells over-express adhesion molecules in response to endothelial injury secondary to turbulent flow. Increased cellular adhesion and associated endothelial dysfunction calls for the recruitment of inflammatory cells, release of cytokines, and deposition of lipids into the atherosclerotic plaque. Atherothrombosis is mediated, in large part, by the inflammatory cascade.1 Enrolled macrophages both release additional cytokines and begin to migrate through the endothelial surface into media of the vessel. This process is further enhanced by the local release of monocyte-colony stimulating factor (M-CSF), which causes monocytic proliferation; local activation of monocytes leads to both cytokine-mediated progression of atherosclerosis and oxidation of low-density lipoprotein (LDL). Once initiated, many mediators of inflammation have been described to influence the development of the atherosclerotic plaque.2 Inflammatory mediators expressed by smooth cells within the atherosclerotic plaque include interleukin (IL)-1β, tumor necrosis factor (TNF)-α and β, IL-6, M-CSF, monocyte chemotactic protein-1 (MCP-1), and IL-18. The impact of these mediators is various and includes mitogenesis, intracellular matrix proliferation, and angiogenesis and foam cell development.3 Gliptins are an innovative class of oral anti-diabetic agents that enhance and prolong the physiological actions of incretin hormones that increase insulin secretion. Sitagliptin is an orally available dipeptidyl peptidase-IV inhibitor (DPPI) developed to be used as a once-daily treatment for type 2 diabetes mellitus and has shown beneficial effects on glycemic control, reducing HbA1c and preventing hypoglycemia, as well as on islet mass and function with no significant adverse effects.4,5 In addition to glucose control by insulin and glucagon secretion, incretins improve peripheral insulin sensitization, cardiac and neuronal protection and beta-cell preservation. The use of an incretin enhancer (such as sitagliptin) might present beneficial effects on diabetes pathophysiology and on prevention of its serious complications like atherosclerosis.
Materials and methods
Animals
A total of 18 local domestic male rabbits were included in this study. The animals were randomly divided into three groups (6 rabbits in each group). Group I rabbits fed normal chow (oxiod) diet for 12 weeks. Group II rabbits fed 1% cholesterol-enriched diet for 12 weeks. Group III rabbits fed with cholesterol-enriched diet for 6 weeks, and then continued on cholesterol-enriched diet and treated with sitagliptin 125 mg/kg/day orally for the next 6 weeks. Blood samples were collected at the start of the study, at 6 weeks of the study, and then at the end of treatment course for measurement of serum lipid profile (total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL)), high-sensitivity C-reactive protein (hsCRP), and TNF-α). At the end of the study, the aorta were removed for measurement of aortic malondialdehyde (MDA), glutathione (GSH) and aortic intima-media thickness, and for sectioning for histopathology.
Preparation of samples
From each rabbit about 3 mL of blood was collected from the central ear artery without use of heparin after an overnight fasting. The blood sampling was done first at the start of the study, that is, at time 0 and after 6 weeks of the induction period, and then at end of the treatment course (12 weeks). The blood samples were allowed to clot at 37°C and centrifuged at 3000 r/min for 15 min. Sera were taken and analyzed for determination of serum TC, TG, HDL-cholesterol (HDL-C), hsCRP, and TNF-α.
Tissue preparation for oxidative stress measurement
An amount of equal to 20% homogenates of tissues was prepared in phosphate buffer at pH 7.5 containing 1 mmol/L sodium-EDTA. The homogenates were centrifuged at 20,000×g at 4°C for 30 min and the supernatants were used for biochemical measurements of GSH and MDA levels.
Histopathological procedure
Autopsy of aortic (abdominal and thoracic aorta) sectioning was done at the end of the study (after 12 weeks); the histopathology was used to confirm the anti-atherogenic effect of sitagliptin in comparison with the control groups (normal control and induced untreated control). The sections were examined by microscope under magnification power of 4×, 10×, and 40×, and the histological changes were determined according to the American Heart Association classification of atherosclerosis,6 which divides atherosclerotic lesions into six types as follows:
Type I (initial) lesion: isolated macrophage foam cells;
Type II (fatty streak) lesion: mainly intracellular lipid accumulation;
Type III (intermediate) lesion: type II changes and small extracellular lipid pools;
Type IV (atheroma) lesion: type II changes and core of extracellular lipid;
Type V (fibro-atheroma) lesion: lipid core and fibrotic layer or multiple lipid cores and fibrotic layers;
Type VI (complicated) lesion: complicated fibro-atheroma with hemorrhage or thrombus.
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM); by using SPSS version 17, unpaired t-test was used to compare the mean values between different groups.
Results
Effect of sitagliptin on serum lipid profile
At end of the first 6 weeks, all animals that had been fed with high cholesterol diet showed significant increase in lipid profile. But at the end of the 12 weeks, sitagliptin-treated group showed significant reduction in serum lipids in comparison to untreated group (see Table 1).
Table 1.
Effect of cholesterol-enriched diet and sitagliptin 125 mg/kg/day on serum lipid profile. The data are expressed as mean ± SEM.
| Group | TC (mg/dL) | TG (mg/dL) | HDL (mg/dL) | ||
|---|---|---|---|---|---|
| I | Normal group | Zero time | 54.3 ± 2.00 | 44.5 ± 0.6 | 16.2 ± 0.25 |
| 6 weeks | 56 ± 1.9 | 44.7 ± 0.9 | 15.8 ± 0.50 | ||
| 12 weeks | 57.2 ± 1.5 | 46.50 ± 1.0 | 16.4 ± 0.30 | ||
| II | Induced untreated group | Zero time | 52.5 ± 2.26 | 45.5 ± 1.8 | 16.7 ± 0.34 |
| 6 weeks | 596 ± 4.5* | 159.3 ± 5.0* | 20.0 ± 0.30* | ||
| 12 weeks | 720 ± 9.5† | 187.8 ± 8.0† | 18.9 ± 0.75† | ||
| III | Sitagliptin-treated group | Zero time | 56.5 ± 2.00 | 47.5 ± 1.5 | 16.9 ± 0.55 |
| 6 weeks | 605 ± 4.9* | 162.8 ± 3.8* | 17.9 ± 0.40* | ||
| 12 weeks | 380 ± 3.2† | 86.0 ± 1.90† | 20.4 ± 0.90† | ||
SEM: standard error of the mean.
p < 0.05 (means at 6 weeks versus means at time zero); †p < 0.05 (means at 12 weeks versus means at 6 weeks).
Effect on aortic tissue reduced GSH level and MDA
At the end of study, after 12 weeks of high cholesterol diet, the aortic GSH level was significantly decreased in the induced untreated group (II) and there was a significant increment in MDA level (p < 0.05) in comparison with the normal control group. For the sitagliptin-treated group (III), after 12 weeks of high cholesterol diet, there was a significant increment in the GSH level (p < 0.05) associated with significant decrement in MDA level (p < 0.05; Table 2).
Table 2.
Changes in aortic oxidative stress (GSH in nmol/mg and MDA in µmol/g) at the end of study. The data were expressed as mean ± SEM.
| Groups | Aortic MDA level (µmol/g) | Aortic GSH level (nmol/mg) | |
|---|---|---|---|
| I | Normal group | 1.9 ± 0.22 | 40.3 ± 2.4 |
| II | Induced untreated group | 9.0 ± 0.56* | 20.9 ± 1.9* |
| III | Sitagliptin-treated group | 2.9 ± 0.42† | 30.3 ± 2.7† |
MDA: malondialdehyde; GSH: glutathione.
p < 0.05 (means at the end of the study for the induced untreated group); †p < 0.05 (means at the end of the study for the sitagliptin treated group).
Effect of sitagliptin on TNF-α and hsCRP
Before the study, the baseline levels of serum hsCRP and TNF-α were statistically not significant among all groups. After 6 weeks of high cholesterol diet, the TNF-α and hsCRP levels significantly increased (p < 0.05) in all groups except the normal group. After 12 weeks, the hsCRP and TNF-α levels significantly decreased in the sitagliptin-treated group (p < 0.05) as compared with the induced untreated group (Table 3).
Table 3.
Effect of cholesterol-enriched diet and sitagliptin 125 mg/kg/day on serum inflammatory marker (TNF-α level in pg/mL and hsCRP in mg/L). The data were expressed as mean ± SEM.
| Group | TNF-α (pg/mL) | hsCRP (mg/L) | ||
|---|---|---|---|---|
| I | Normal group | Zero time | 0.60 ± 0.09 | 3.0 ± 0.0 |
| 6 weeks | 1.08 ± 0.11 | 3.0 ± 0.0 | ||
| 12 weeks | 1.05 ± 0.06 | 3.0 ± 0.0 | ||
| II | Induced untreated group | Zero time | 0.77 ± 0.10 | 3.0 ± 0.0 |
| 6 weeks | 4.70 ± 0.54* | 43 ± 1.8* | ||
| 12 weeks | 7.05 ± 0.44† | 57 ± 3.0† | ||
| III | Sitagliptin-treated group | Zero time | 0.90 ± 0.10 | 3.0 ± 0.0 |
| 6 weeks | 5.52 ± 0.15* | 42 ± 1.6* | ||
| 12 weeks | 2.50 ± 0.20† | 18 ± 1.6† | ||
SEM: standard error of the mean; TNF-α: tumor necrosis factor-α; hsCRP: high-sensitivity C-reactive protein.
p < 0.05 (means at 6 weeks versus means at zero time); †p < 0.05 (means at 12 weeks versus means at 6 weeks.
Effect of sitagliptin on atherosclerosis and aortic intima-media thickness
At the end of 12 weeks of high cholesterol diet, rabbits treated with sitagliptin had a significant reduction in the severity of atherosclerotic lesions in comparison with rabbits in the induced untreated group (Figure 1(b) and (c)). The level of aortic intima-media thickness (measured by histomorphometry) was significantly increased in the induced untreated group (II) in comparison with normal control (p < 0.05) as shown in Figure 1(a) and (b). The aortic intima-media thickness level of sitagliptin-treated group (III) was significantly lower than that of the induced untreated group (II) as shown in Figure 1 and Table 4.
Figure 1.
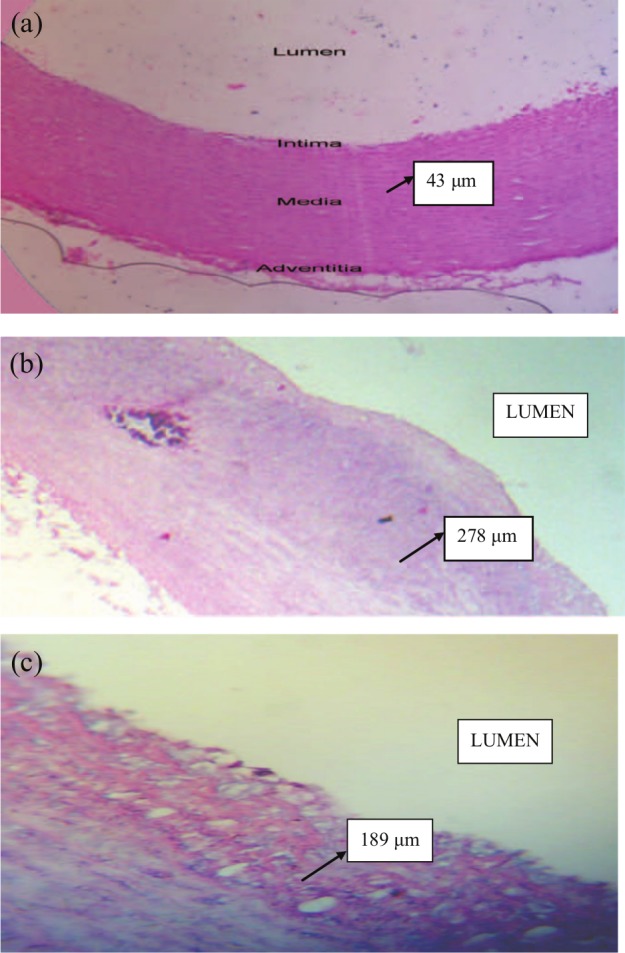
The photomicrograph of histomorphometric section in aortic arch of (a) normal, (b) hyperlipidemic, and (c) sitagliptin hyperlipidemic rabbits shows the difference in aorta intima-media thickness. The section stained with hematoxylin and eosin (4×): (a) image showing normal appearance of arterial wall layers; (b) image showing a fibro-atheromatous plaque with thick layers of fibrous connective tissue overlying a largely necrotic, fatty mass—advance atherosclerotic lesion (Type IV); and (c) image showing significant decrease in the aortic intima thickness as compared to induced untreated (b) group.
Table 4.
Changes in aortic intima-media thickness in (µm) at the end of the study. The data expressed as mean ± SEM.
| Groups | Aortic intima-media thickness (µm) | |
|---|---|---|
| I | Normal group | 47.0 ± 2.7 |
| II | Induced untreated group | 289 ± 53.7* |
| III | Sitagliptin-treated group | 204 ± 22.35† |
SEM: standard error of the mean.
p < 0.05 (means at end of the study for the induced untreated group); †p < 0.05 (means at end of the study for the sitagliptin-treated group).
Discussion
In this study, we demonstrate that high atherogenic diet causes significant increment in lipid parameter7,8 (TC, TG, and atherogenic index) in comparison with the control group. Treatment with sitagliptin causes significant reduction in (TC, TG, and atherogenic index) in comparison with the induced untreated group. This result is consistent with those reported by Matikanianin et al.9 In this study, sitagliptin treatment significantly reduced the elevation of inflammatory markers (hsCRP and TNF-α) in atherosclerosis model of hypercholesterolemic rabbit,10,11 suggesting that sitagliptin inhibits vascular inflammation induced by high atherogenic diet. These results are consistent with those reported by Ferreira et al.12 In our study, atherosclerosis was associated with an increase in the levels of the lipid peroxidation product MDA and a decrease in the level of GSH in aortic tissue, suggesting an increase in the levels or activity of oxygen radicals. MDA and GSH have been considered as specific indicators of oxidative status.13 MDA level is widely utilized as a marker of lipid peroxidation and its measurement gives a direct evidence for LDL oxidation and is important in predicting free radical–induced injury. Therefore, the observed elevation in tissue MDA may be attributed to hyperlipidemia that enhances the processes of lipid peroxidation. Hypercholesterolemia could increase the levels of reactive oxygen species (ROS) through stimulation of polymorph-nuclear leukocytes (PMNLs) and dysfunction of endothelial cells.14,15 Furthermore, hypercholesterolemia, especially if prolonged, results in vascular oxidant burden,16,17 which could favor GSH depletion because of enhanced oxidation of the tripeptide or its consumption by electrophilic compounds like lipoperoxidation aldehydes.18,19 Sitagliptin treatment had significantly reduced aortic MDA level, suggesting decrease in ROS and subsequent lipid peroxidation. Also sitagliptin had a significant effect on aortic GSH levels where it prevents GSH depletion in hypercholesterolemic rabbit, and thus maintains antioxidant reserve which is important for vascular protection against lipid peroxide.12 In rabbits treated with sitagliptin, there was a significant reduction in the severity of atherosclerotic lesions in comparison with rabbits in the induced untreated group. Also there is significant decrement in aortic intima-media thickness (p < 0.05) in the sitagliptin-treated group compared with that of the induced untreated group. In our study, we found that sitagliptin exert anti-inflammatory effect by reducing hsCRP and TNF-α and had antioxidant effect by reducing lipid peroxide (MDA) and enhancing GSH. Sitagliptin has potent and rapid anti-inflammatory effect by which it may reduce atherosclerosis.20 Our findings are consistent with those of Barbieri et al.21 who found that dipeptidyl peptidase inhibitors decrease inflammation and oxidative stress in diabetic patients and therefore decrease progression of atherosclerosis. Our results may provide answers about how sitagliptin reduces aortic intima-media thickness and atherosclerosis by suppression of systemic inflammatory response and oxidative stress.
Conclusion
The results of this study reveal that sitagliptin reduces atherosclerosis progression in experimentally induced atherosclerosis by interfering with inflammatory and oxidative pathways.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Libby P. Inflammation in atherosclerosis. Nature 2002; 420(6917): 868–874. [DOI] [PubMed] [Google Scholar]
- 2. Schonbeck U, Sukhova GK, Shimizu K, et al. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc Natl Acad Sci U S A 2000; 97(13): 7458–7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mallika V, Goswami B, Rajappa M. Atherosclerosis pathophysiology and the role of novel risk factors: a clinicobiochemical perspective. Angiology 2007; 58(5): 513–522. [DOI] [PubMed] [Google Scholar]
- 4. Penfornis A, Borot S, Raccah D. Therapeutic approach of type 2 diabetes mellitus with GLP-1 based therapies. Diabetes Metab 2008; 34(2): S78–S90. [DOI] [PubMed] [Google Scholar]
- 5. Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patient with type 2 diabetes. Diabetes Res Clin Pract 2008; 79(2): 291–298. [DOI] [PubMed] [Google Scholar]
- 6. Stary HC, Chandler AB, Dinsmore ER, et al. A definition of advanced types of atherosclerotic lesion and a histological classification of atherosclerosis. Circulation 1995; 92: 1355–1374. [DOI] [PubMed] [Google Scholar]
- 7. Howard HT, Culley NC. Accumulation of low density lipoprotein associated cholesterol in calcifying vesicle fractions correlates with intimal thickening in thoracic aortas of juvenile rabbits fed a supplemental cholesterol diet. Lipids Health Dis 2006; 5: 5–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bauersachs J, Hiss K, Fraccarollo D, et al. Simvastatin improves left ventricular function after myocardial infarction in hypercholesterolemic rabbits by anti-inflammatory effects. Cardiovasc Res 2006; 72: 438–446. [DOI] [PubMed] [Google Scholar]
- 9. Matikanianin N, Manttari S, Schwerzer A, et al. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patient with type 2 diabetes. Diabetologia 2006; 49: 2049–2057. [DOI] [PubMed] [Google Scholar]
- 10. Zhang D, Che D, Zhao S, et al. Effects of atorvastatin on C-reactive protein secretions by adipocytes in hypercholesterolemic rabbits. J Cardiovasc Pharmacol 2007; 50: 281–285. [DOI] [PubMed] [Google Scholar]
- 11. Zhao S, Wu Z. Atorvastatin reduces serum leptin concentration in hypercholesterolemic rabbits. Clin Chim Acta 2005; 360: 133–140. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira L, Teixeira-de-Lemos E, Pinto F, et al. Effect of sitagliptin treatment on dysmetabolism, inflammation, and oxidative stress in animal model of type 2 diabetes (ZDF rat). Mediators Inflamm 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayne ST. Antioxidant nutrient and chronic disease: use of biomarker of exposure and oxidative stress status in epidemiologic research. J Nutr 2003; 133: 933–940. [DOI] [PubMed] [Google Scholar]
- 14. Prasad K. Regression of hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Atherosclerosis 2008; 197: 34–42. [DOI] [PubMed] [Google Scholar]
- 15. Prasad K. Hypocholesterolemic and antiatherosclerotic effect of flax lignan complex isolated from flaxseed. Atherosclerosis 2005; 179: 269–275. [DOI] [PubMed] [Google Scholar]
- 16. Mugge A, Brandes RP, Boger RH, et al. Vascular release of superoxide radicals is enhanced in hypercholesterolemic rabbits. J Cardiovasc Pharmacol 1994; 24: 994–998. [DOI] [PubMed] [Google Scholar]
- 17. Napoli C, Lerman LO. Involvement of oxidation-sensitive mechanisms in the cardiovascular effects of hypercholesterolemia. Mayo Clin Proc 2001; 76: 619–631. [DOI] [PubMed] [Google Scholar]
- 18. Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol 2000; 20: 1430–1442. [DOI] [PubMed] [Google Scholar]
- 19. Griffith OW. Biological and pharmacological regulation of mammalian glutathione synthesis. Free Radical Biol Med 1999; 27: 922–935. [DOI] [PubMed] [Google Scholar]
- 20. Makdissi A, Ghanim H, Vora M, et al. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab 2012; 97(9): 3333–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbieri M, Rizzo MR, Marfella R, et al. Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis 2013; 227(2): 349–354. [DOI] [PubMed] [Google Scholar]


