Figure 6.
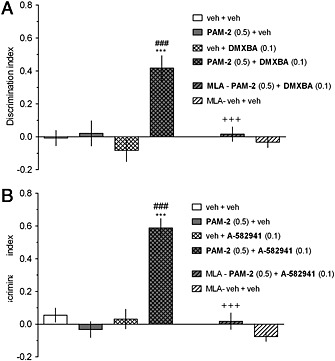
The co‐administration of inactive doses of PAM‐2 with either DMXBA (a) or A‐582941 (b) facilitates performance in the NORT. PAM‐2 (0 or 0.5 mg·kg−1) was co‐injected with DMXBA (0 or 0.1 mg·kg−1) or A‐582941 (0 or 0.1 mg·kg−1) 30 min before T1 (acquisition trial). T2 (retention trial) was performed 24 h after T1. Data are shown as the mean ± SEM of discrimination index (DI) during T2. Symbols: ***p<0.001 significant increase in DI compared to that for the vehicle‐treated group; ###p<0.001 significant increase in DI compared to that for the vehicle+DMXBA (a)‐ or vehicle+A‐582941 (b) ‐ group. +++p<0.001, significant reduction in DI compared to that for the PAM‐2+DMXBA (a)‐ or PAM‐2+A‐582941 (b)‐treated group.
