Figure 3.
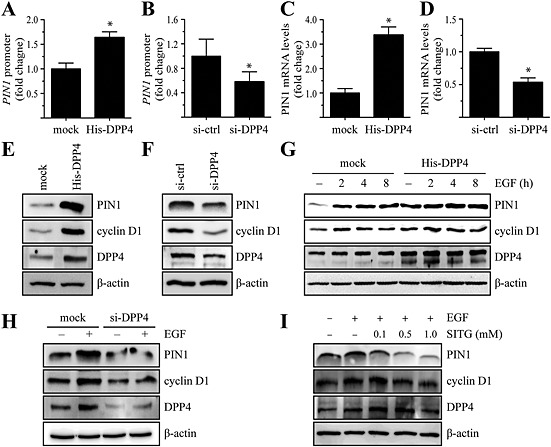
DPP4 regulates PIN1 expression via activation of E2F1 in MCF7 cells. (A and B) The PIN1‐luc luciferase promoter‐reporter construct and the pRL‐TK (Renilla luciferase control reporter) vector were cotransfected with constructs expressing histidine‐tagged DPP4 (His‐DPP4) (A) or DPP4‐specific siRNA (siRNA‐DPP4) (B). After 48 h, the firefly luciferase activity was determined in cell lysates and normalized to Renilla luciferase activity. Columns represent the means ± SD of triplicate samples.*P < 0.05, significantly different from control cells. (C and D) Cells were transfected with His‐DPP4 (C) or siRNA‐DPP4 (D). Levels of PIN1 mRNA were assessed by real‐time PCR analysis. The signal intensity corresponding to each mRNA was densitometrically determined and normalized to GAPDH mRNA. Columns represent the means ± SD of triplicate measurements from two experiments. *P < 0.05, significantly different from mock‐transfected (C) or siRNA‐control‐transfected (D) cells. (E and F) Cells were transfected with His‐DPP4 (E) or siRNA‐DPP4 (F). At 48 h after transfection, the cells were harvested and lysed. Proteins in whole cell lysates were separated by SDS‐PAGE and immunoblotted. (G) Cells were transfected with His‐DPP4 or mock transfected, serum starved, treated with 1 ng∙mL−1 EGF for the indicated times, harvested and lysed. Proteins in whole cell lysates were separated by SDS‐PAGE and immunoblotted. (H) siRNA‐control or siRNA‐DPP4 constructs were transfected into MCF7 cells. At 48 h after transfection, the cells were serum starved, treated with 1 ng∙mL−1 EGF for the indicated time, harvested and lysed. Proteins in whole cell lysates were separated by SDS‐PAGE and immunoblotted. (I) Cells were serum starved, treated with the indicated concentration of sitagliptin (SITG) for 24 h, then exposed or not exposed to 1 ng∙mL−1 EGF for 8 h, harvested and lysed. Proteins in whole cell lysates were separated by SDS‐PAGE and immunoblotted.
