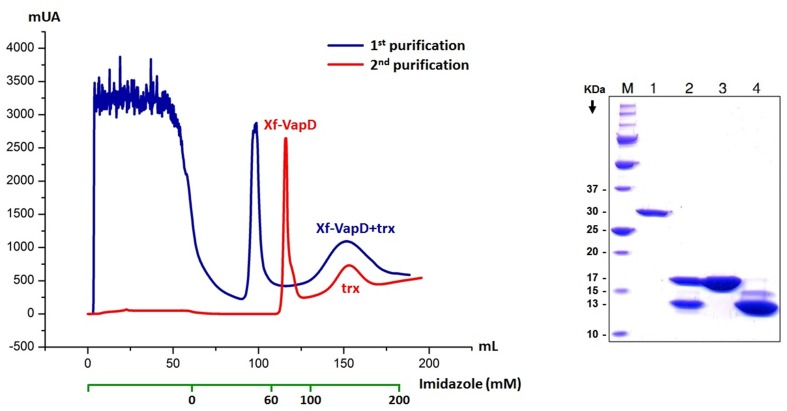
Fig 3. Purification of recombinant Xf-VapD.
A chromatogram showing the two steps used in the purification of Xf-VapD: Xf-VapD fused with a trx-tag was obtained first, and then trypsin cleavage was used to remove the trx-tag. On the right, a 12.5% SDS-PAGE is shown for the Xf-VapD purification process. The lanes are as follows: M, protein marker; 1, Xf-VapD fused to trx-tag (31 kDa); 2, Xf-VapD and trx-tag after cleavage with trypsin; 3, totally purified Xf-VapD (16.5 kDa); and 4, trx-tag (~13 kDa). The gel was stained using Coomassie brilliant blue.