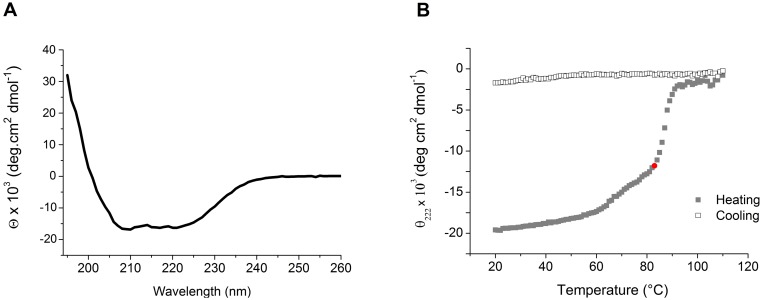
Fig 4. Secondary structure and thermo stability assay of Xf-VapD.
(A) Circular dichroism spectrum (200 nm–260 nm) of the recombinant purified Xf-VapD. The secondary structure of the protein was found to be 69% α-helices, 19% β-sheets, and 12% random coils. (B) Thermal unfolding/refolding of Xf-VapD. At 83°C during the heating phase, 50% of the proteins were unfolded (red marker). The conformational structure of the protein was not restored during the cooling phase.