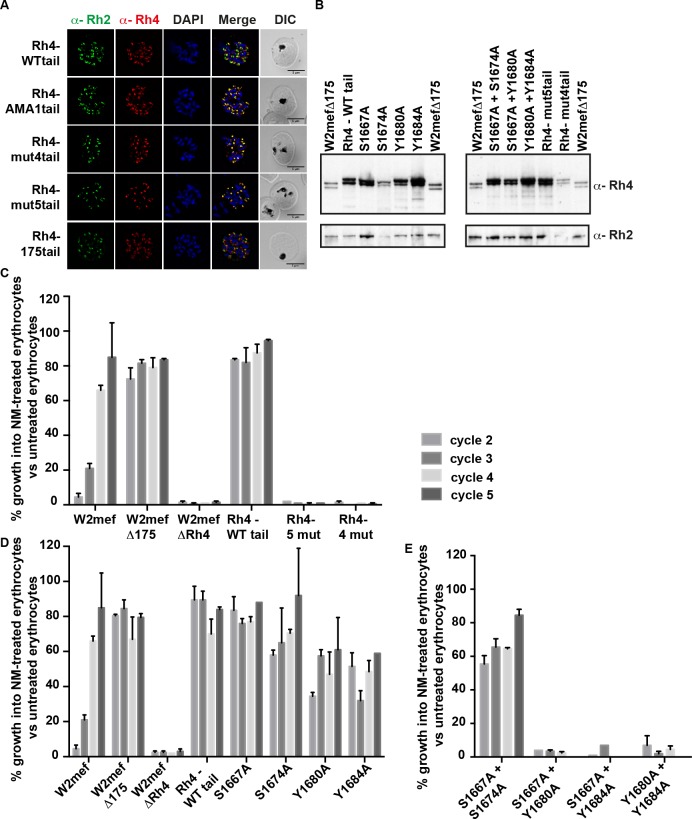
Fig 5. Specific amino acid residues in the cytoplasmic domain are essential for PfRh4 function.
(A) Localization of PfRh4 and PfRh2 in Rh4-WT tail, RH4-AMA1tail, Rh4-mut4tail and RH4-mut5tail lines as detected using anti-PfRh4 monoclonal and anti-PfRh2 polyclonal antibodies. Parasite nuclei were stained with DAPI. (B) Expression of PfRh4 and PfRh2 in W2mefΔ175 and all transgenic lines as detected by anti-PfRh4 and anti-PfRh2 antibody. All transgenic lines migrated as a slightly larger doublet compared to PfRh4 in W2mefΔ175, consistent with the addition of the hexa-histidine tag at the C-terminus of the protein. (C) Growth assays of transgenic lines with four and five mutations at serine and tyrosine amino acid residues in the PfRh4 cytoplasmic tail. (D) Growth assays of single mutations in the PfRh4 cytoplasmic tail. (E) Growth assays of double mutations in the PfRh4 cytoplasmic tail. In all three panels, parasitaemia was measured in neuraminidase-treated, and untreated erythrocytes after every 48 hours incubation (labelled as cycles). The parasite lines used in this experiment are displayed on the X-axis. The y-axis represents parasitaemia of neuraminidase-treated erythrocytes as a percentage of parasitaemia of the same line grown on untreated erythrocytes. Error bars represent +1 standard error of the mean. Assay performed three times on separate days, each in triplicate.