Fig. 1.
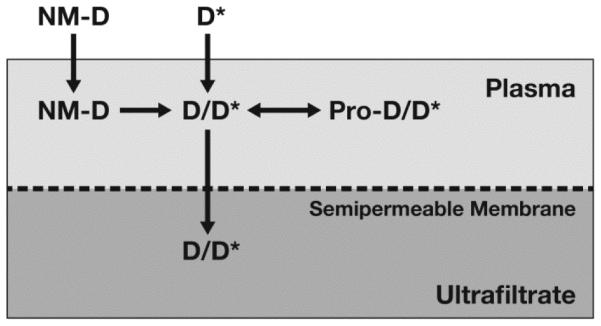
Stable isotope drug release method. In this method, the stable isotopically labeled drug (D*) equilibrates with protein (Pro) and formulation components identical to the unlabeled, normoisotopic drug (D) released from the nanomedicine (NM) formulation. Therefore, the ultrafilterable fraction of the isotopically labeled drug represents a reliable measurement of free drug fraction, and plasma protein bound fraction can be calculated from equation (i) in the text. The unencapsulated and encapsulated nanomedicine fractions can then be easily calculated, using equations (ii) and (iii) in the text, respectively.
