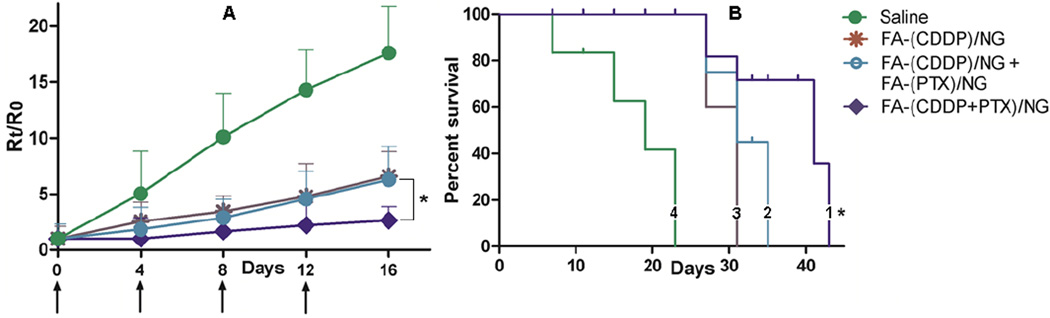
Figure 7.
In vivo antitumor efficacy of FA-(CDDP+PTX)/NG vs. FA-(CDDP)/NG + FA-(PTX)/NG co-administration against A2780/Luc human ovarian cancer xenografts. (A) Comparison of tumor growth inhibition as indicated by relative radiance units over time following IV administration of FA-(CDDP+PTX)/NG (◆) or FA-CDDP/NG + FA-PTX/NG or FA-(CDDP)/NG (Ж) or control (●). Drug formulations were injected in 100 µL at a dose of 4 mg CDDP or 1 mg PTX equivalents/kg body weight 4 times at 4-day intervals as indicated by the arrows. Values indicated are means ± SD (n=8). (C) Kaplan–Meier analysis of overall survival in FA-(CDDP+PTX)/NG (1) or FA-(CDDP)/NG + FA-(PTX)/NG group (2) or FA-(CDDP)/NG (3) or control group (4). * P < 0.05. Log-rank test was used to determine statistical difference between FA-(CDDP+PTX)/NG and FA-(CDDP)/NG + FA-(PTX)/NG groups, *P < 0.05.