Abstract
Thymosin beta 4 (Tβ4), a secreted 43 amino acid peptide, promotes oligodendrogenesis, and improves neurological outcome in rat models of neurological injury. We demonstrated that exogenous Tβ4 treatment up-regulated the expression of the miR-200a in vitro in rat brain progenitor cells and in vivo in the peri-infarct area of rats subjected to middle cerebral artery occlusion (MCAO). The up-regulation of miR-200a down-regulated the expression of the following targets in vitro and in vivo models: (1) growth factor receptor-bound protein 2 (Grb2), an adaptor protein involved in EGFR/Grb2/Ras/MEK/ERK1/c-Jun signaling pathway, which negatively regulates the expression of myelin basic protein (MBP), a marker of mature oligodendrocyte; (2) ERRFI-1/Mig-6, an endogenous potent kinase inhibitor of epidermal growth factor receptor (EGFR), which resulted in activation/phosphorylation of EGFR; (3) Friend of GATA 2 (FOG2), and phosphatase and tensin homolog deleted in chromosome 10 (PTEN), which are potent inhibitors of the PI3K/AKT signaling pathway, and resulted in marked activation of AKT and, (4) transcription factor- p53, which induces pro-apoptotic genes, and possibly reduced apoptosis of the progenitor cells subjected to oxygen glucose deprivation (OGD). Anti-miR-200a transfection reversed all the effects of Tβ4 treatment in vitro. Thus, Tβ4 up-regulated MBP synthesis, and inhibited OGD-induced apoptosis in a novel miR-200a dependent EGFR signaling pathway. Our findings of miR-200a–mediated protection of progenitor cells may provide a new therapeutic importance for the treatment of neurological injury.
Keywords: Thymosin beta 4, MicroRNA, epidermal growth factor receptor (EGFR), growth factor receptor-bound protein 2 (GRB2), AKT, phosphatase and tensin homologue (PTEN), p53, myelin basic protein (MBP), Progenitor cells, oligodendrocytes
Graphical abstract
Introduction
Thymosin β4 (Tβ4) is a secreted 43-amino acid G-actin sequestering peptide (Goldstein AL 1966) which regulates the cellular actin-cytoskeleton and cell migration (Huff et al. 2001, Sanders et al. 1992). Tβ4 has multiple biological functions including inhibiting inflammation and promoting regeneration in both dermal and cardiac injury models (Goldstein et al. 2005). In addition, Tβ4 induces differentiation of oligoprogenitor cells (OPC) into mature myelin secreting oligodendrocytes in vitro and in vivo in experimental animal models of multiple sclerosis (MS), embolic stroke and traumatic brain injury (TBI) (Morris DC 2010a, Xiong Y 2010, Zhang J 2009, Santra M 2012, Santra M 2014). In all three animal models, Tβ4 treatment improves functional outcome. This improvement may in-part be attributed to migration of OPCs towards the site of injury and the differentiation of OPCs which remyelinates injured axons (Aguirre A 2007a, Zhang L 2010, Menn B 2006, Morris DC 2010a, Zhang C 2010, Jiang Q1 2006, Zhang L 2013, Zhang R 2013). Neurorestorative therapies, including Tβ4 for stroke, regulate multiple molecular mechanisms during the recovery phase of neurological injury. These molecular mechanisms are involved in remodeling of the injured brain via angiogenesis, neurogenesis, and axonal and dendritic plasticity, and thereby enhance neurological recovery (Zhang & Chopp 2009, Morris DC 2010b). To aid in the translation of neurorestorative therapies to the clinical for stroke, it is important to elucidate the molecular mechanisms that regulate neurological recovery.
The adult subventricular zone (SVZ) normally produces neuronal progenitor cells (NPCs) and OPCs. Stroke increases production of newborn NPCs and OPCs of which very few survive (Arvidsson A 2002, Zhang R 2013). Increasing the survival of newborn NPCs and OPCs, and their differentiation into neurons and oligodendrocytes, respectively, may be a therapeutic approach for the treatment of diseases associated with ischemic brain injuries. Increasing evidence suggests that the microRNAs (e.g. miR-200, 124a, 133b, 17–92 cluster, 29a and 146a) regulate proliferation and differentiation of adult neural progenitor cells (NPCs) and OPCs (Lee ST 2010, Rink C 2011, Wang C 2014, Ouyang YB 2014, Liu XS 2013b, Xin H 2013, Liu XS 2013a, Zhang Y 2013, Liu XS 2011, Ouyang YB 2013, Santra M 2014). Based on Agilent Mouse microRNA Microarray analysis of ischemic mouse brain tissue samples, the miR-200 family (miR-200a, miR-200b, miR-200c, miR-141, and miR-429) members (miR-200) show the best neuroprotective effect against oxygen glucose deprivation (OGD) among the microRNAs (Lee ST 2010). In addition, NPCs undergo apoptosis in the absence of miR-200 in mouse and zebra fish brain (Choi PS 2008).
Our previous data demonstrated that Tβ4 treatment induces myelin basic protein (MBP) synthesis, and differentiation of OPCs into oligodendrocytes, by up-regulating p38 MAPK in the cells isolated from the SVZ; and in a miR-146a dependent pathway in an OPC cell line-N20.1a and primary OPCs (Santra M 2014, Santra M 2012). Enhanced EGFR signaling induces remyelination of axons of injured neurons, and increases migration of newly generated NPCs from the SVZ to the olfactory bulb (OB) via the rostral migratory stream (Adam L 2009, Aguirre A 2007a, Aguirre A 2005). The miR-200 family members are highly expressed in the OB and are required for terminal differentiation of NPCs (Choi PS 2008). The miR-200 not only induces terminal differentiation of NPCs in the OB, but also induces neuronal differentiation in neuronal stem cells and in a PC-12 cell line (Pandey A 2015). Based on TargetScan analysis (http://www.mirbase.org), we identified multiple target sequences for the miR-200 in adaptor and mediators of the EGFR signaling e.g. Mig-6/ ERRFI-1, growth factor receptor-bound protein 2 (Grb2), Friend of GATA 2 (FOG2), phosphatase and tensin homolog deleted in chromosome 10 (PTEN). We therefore sought to investigate the effect of Tβ4 treatment on miR-200 in the regulation of EGFR expression, and EGFR signaling.
In the current study, we investigated the molecular mechanism of Tβ4-mediated differentiation of OPCs into myelinating mature oligodendrocytes and their protection from OGD-mediated apoptosis. We found that Tβ4 enhanced EGFR signaling in a miR-200a dependent pathway in progenitor cells. Tβ4 treatment (in vitro and in vivo) up-regulated miR-200a, and down-regulated the known targets of miR-200a e.g. ERRFI-1/Mig-6, Grb2, FOG2, PTEN and p53 which were involved in MBP synthesis, AKT activation, cell survival and protection of progenitor cells from OGD-induced cell death. Up-regulation of miR-200a after Tβ4 treatment induced oligodendrogenesis in a cell culture model under condition of OGD. Our findings of an important role of miR-200a in mediating progenitor cell and oligodendrocyte protection may provide a new therapeutic target for the treatment of neurological injury.
Materials and Methods
All animal experiments were performed according to protocols approved by the Henry Ford Hospital Institutional Animal Care and Use Committee.
Middle cerebral artery occlusion (MCAO)
Male Wistar rats (300–400 grams) purchased from Charles River Breeding Company (Wilmington, MA, USA), were anesthetized with 1.0–2.0% isoflurane in 70% N2O and 30% O2 using a face mask. Right MCAO was performed according to a modified protocol of intraluminal vascular occlusion established in our laboratory (Santra M 2006a, Santra M 2006b).
Isolation of neuroprogenitor cells (NPCs) from SVZ
The SVZ of the adult male Wistar rat (Charles river Breeding company, Wilmington, MA, USA), was examined under a microscope (Olympus BX40; Olympus Optical, Tokyo, Japan) and was surgically dissected according to methods previously described (Santra M 2006a, Santra M 2006b). SVZ cells were dissociated and cultured in neurosphere growth medium containing epidermal growth factor (EGF) and bFGF (20ng/ml), as previously described (Santra M 2006a, Santra M 2006b). The generated neurospheres were passed by mechanical dissociation and reseeded as single cells at a density of 104 cells/cm2 in EGF-containing media. Passage 2 and 3 neurospheres were used in the present study.
Preparation of primary rat embryonic oligodendrocyte progenitor cells (OPCs)
Primary rat embryonic oligodendrocyte progenitor cells (OPCs) were isolated and prepared according to the method of Chen et al. (Chen Y 2007). Briefly, on embryonic day 17, the rat embryos were removed from a pregnant Wistar rat in a laminar flow hood. The cortices were dissected and dissociated with 0.01% trypsin and DNase at 37°C for 15 min followed by platting with DMEM containing 20% fetal bovine serum (FBS) in poly-D-lysine coated T75 cell culture flasks (∼107/flask) for 10 days. These confluent cells were shaken at 200 rpm at 37°C for 1 h to remove microglial cells in the medium and further shaken at 200 rpm at 37°C for 12 h to collect OPCs in the medium. The collected OPCs from medium were cultured in Poly-D, L-ornithine-coated Petri dishes at a cell density of 104 per/cm2 with OPC medium containing platelet derived growth factor (PDGF) and basic fibroblast growth factor (bFGF) (10ng/ml).
Tβ4 treatment
The cells (102 cells/cm2) were treated with 0, 25, or 50 ng/ml of Tβ4 (RegeneRx Biopharmaceuticals Inc, Rockville, MD) for 7 days. For the treatment with kinase inhibitors, the cells were pretreated EGFR specific inhibitor-AG-1478 at the dose of 1–3 nM (Calbiochem, San Diego, CA, USA) for 20–30 min before the addition of Tβ4 into the medium, as previously described (Moscatello DK 1998). For in vivo treatment in rats, Tβ4 (12mg/kg) was intraperitoneally injected 24h after MCAO. The injection was continued every 3 days for 4 additional doses (12mg/kg), as previously reported (Morris DC 2014).
Transfection
The cells (106) were mixed with 1 µg plasmid DNA or 100 pmol of siRNA/oligo nucleotides and pulsed according to the manufacturer’s instruction. The transfected cells were immediately plated into Petri dishes with DMEM containing 1% FBS and incubated at 37°C for 2 days. A random mixture of oligonucleotides (Ambion, Grand Island, NY, USA) was used as a scrambled oligo control ((Izadi H 2007) for both transfections with Tβ4siRNA (Santa Cruz Biotechnology, Dallas, Texas) and anti-miR™ miRNA inhibitors for miR-200a, −200b, −200c, −429 and −141 (Applied Biosystem, Grand Island, NY, USA). The cells were transfected with the anti-miR™ miRNA inhibitors for miR-200a, −200b, −200c, −429 and −141 according to manufacturer’s protocol (Applied Biosystem, Grand Island, NY, USA) in order to knockdown the expression of miR-200a, −200b, −200c, −429 and −141. The control lentiviral vector without insert was used as a mock transfected control for miR-200 expression vector transfection. In order to over-express miR-200, the cells were transfected with lentiviral vector of miR-200 cluster genes e.g. Lenti-miR-200b-200a-429 and Lenti-miR-200c-141 (addgene, Cambridge, MA, USA), as previously reported (Santra M 2008, Gregory PA 2008).
Quantification of mature miRNAs by real-time qrtPCR
The cDNA for each miRNA and TaqMan assay were performed in triplicate according to the manufacturer’s protocol specified in Applied Biosystems ViiA™ 7 Real-Time PCR System (Applied Biosystem, Grand Island, NY, USA), as previously described (Santra M 2014). Briefly, total RNA was isolated with TRIzol (Qiagen). The microRNA cDNA was performed by individual reverse transcription in the following thermal cycle 16°C for 30 min, 42°C for 30 min, 85°C for 5 min, and then hold at 4°C for TaqMan assays. TaqMan assay was performed in 20µl TaqMan real-time PCR reactions. All values were normalized to U6 snRNA TaqMan miRNA control assay (Applied Biosystem, Grand Island, NY, USA), as the endogenous control. Values obtained from three independent experiments were analyzed relative to gene expression data using the 2−ΔΔCT method (Livak & Schmittgen 2001).
Quantitative analysis of Western blot
Total protein extracts from the cells were prepared, as previously described (Santra et al. 2011). The protein extracts were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis for Western blot analysis. For Western blot analysis, rabbit antiserum for MBP (1:200; Dako, Carpinteria, CA, USA), monoclonal antibodies (1:500) for EGFR and phosphorylated EGFR; monoclonal antibodies (1:1000) for phosphorylated ERK1, c-JUN and FOG-2 (Santa Cruz Biotechnology, Dallas, Texas, USA); rabbit polyclonal antibodies (1:500) for Grb2, Mig-6, Pten, p53 (Cell Signaling Technology, Danver, MA, USA) and mouse monoclonal α-tubulin antibodies (1:5000; Sigma, St Louis, Mo) were used. Donkey anti-goat, anti-rabbit, and anti-mouse horseradish peroxidase (1: 5000; Jackson ImmunoResearch Labs, West Grove, PA, USA) were used as secondary antibodies. Each experiment was repeated at least five times. The protein bands were quantified based on histogram analysis relative to gel loading marker α-tubulin at least 5 independent experiments.
Statistical analysis
Data were summarized using mean and standard deviation. To compare the differences between cell cultures with Tβ4 treatment and without, a one sample t-test or a two-sample t-test was used. For the comparisons of qrtPCR of mRNA/GAPDH, and qrtPCR of miRNA/U6, controls were normalized to 1, so that one-sample t-test was used for analysis. To compare the percentage of positive stained cells out of the total number of cells between Tβ4 treatment and control, a two-sample t-test was used. P-value <0.05 was considered significant.
Results
To examine cell survival of newborn SVZ progenitor cells after stroke, we isolated and cultured progenitor cells as neurospheres from the SVZ of adult rats,- subjected to MCAO for 7 days. Since MCAO stimulates newly generated progenitor cells which are vulnerable to ischemic insults (Zhang R 2013, Zhang R 2004b, Arvidsson A 2002, Zhang R 2004a, Zhang L 2010, Zhang 2011, Zhang RL 2001a, Zhang 2012), we utilized these progenitor cells (cultured as neurospheres) from the SVZ of adult rats, subjected to MCAO for 7 days in all the following experiments. Additionally, normal rat embryonic primary OPCs (Santra M 2012, Santra M 2014) were also employed to determine OPC differentiation into oligodendrocytes and their survival from OGD in subsequent experiments. We sought to investigate whether Tβ4 treatment protects newly generated SVZ progenitor cells from OGD insult and the molecular mechanisms underlying this neuroprotection.
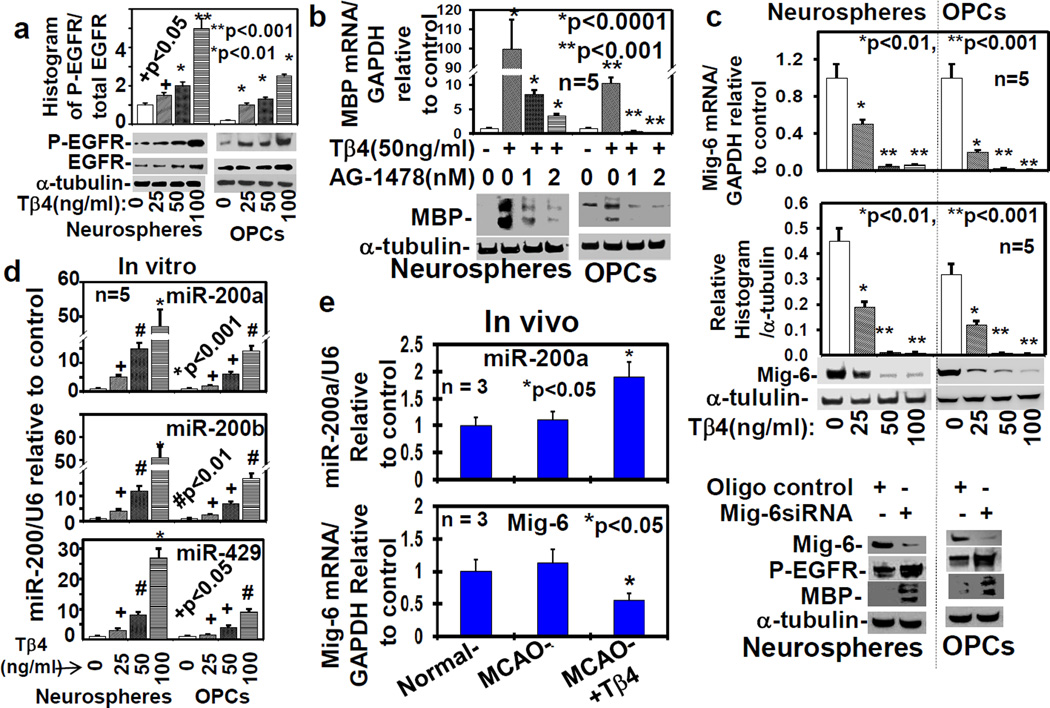
Tβ4 treatment up-regulates expression and phosphorylation/activation of EGFR in rat SVZ neurospheres/progenitor cells and primary OPCs
To determine the effect of Tβ4 on EGFR expression, the neurospheres were cultured from the SVZ of adult rats (n=5), subjected to MCAO for 7 days, and primary embryonic OPCs were isolated from the embryos of pregnant Wistar rats (n=5). These neurospheres and OPCs were treated with 0, 25, 50 and 100ng/ml Tβ4 for 7 days in five independent experiments. The qrtPCR and Western blot data revealed that Tβ4 treatment significantly up-regulated EGFR expression and its phosphorylation at mRNA and protein levels in a dose dependent manner (Fig. 1a and S1a, b). These data demonstrated that Tβ4 treatment induced EGFR signaling. Since EGFR signaling enhances OPC differentiation into mature oligodendrocyte (Doetsch F 2002, Aguirre A 2007a, Scafidi J 2014, Aguirre A 2007c, Aguirre A 2010), we investigated whether EGFR signaling was involved in Tβ4-mediated OPC differentiation into mature oligodendrocyte.
Fig. 1. Tβ4 treatment induces EGFR phosphorylation/activation and the expression of MBP, Mig-6 and miR-200.
a) Quantitative histogram analysis of Western blot for phosphorylation of EGFR (P-EGFR) in the adult rat SVZ neurospheres/progenitor cells (Neurospheres) and rat embryonic primary OPCs (OPCs) after the treatment with Tβ4 (0-100ng/ml) in five independent experiments (n=5). b) Treatment with EGFR specific inhibitor, AG1478, reversed Tβ4-induced MBP expression measured by qrtPCR and Western blot analysis. c) Mig-6 expression were analyzed by qrtPCR (top) and Western blot which were quantified by histogram analysis (bar-graph) in photoshop after the treatment with different dose of Tβ4; and by Western blot after transfection with scrambled oligo control (oligo control), and Mig-6siRNA, in the adult rat SVZ neurospheres/progenitor cells (Neurospheres) and primary rat embryonic OPCs (OPCs), in five independent experiments (n=5). Loading of the samples were normalized with α-tubulin. The mature miR-200 was analyzed in vitro (d) in the adult rat SVZ neurospheres/progenitor cells (Neurospheres) and primary rat embryonic OPCs (OPCs) in five independent experiments (n=5); and mature miR-200a and Mig-6 mRNA were analyzed in vivo (e) in normal rats and rats subjected to MCAO treated with/without Tβ4 (12mg/kg; n = 3). The p-value<0.05, was considered, as significance difference.
EGFR signaling is required for MBP synthesis and OPC differentiation into mature oligodendrocytes
To determine the involvement of EGFR signaling in OPC differentiation into mature oligodendrocyte, EGFR signaling was blocked by tyrphostin AG-1478, a specific pharmaceutical inhibitor of EGFR kinase (Moscatello DK 1998). To examine Tβ4-mediated OPC differentiation, a potent marker of mature oligodendrocyte, MBP was measured (Santra M 2014, Santra M 2012) in rat SVZ-neurospheres and primary OPCs after the treatment with 50ng/ml Tβ4 with/without AG-1478 for 7 days. The qrtPCR and Western blot data revealed that Tβ4 treatment markedly up-regulated mRNA and protein levels of MBP. In contrast, AG-1478 treatment reversed this Tβ4 effect on up-regulation of MBP (Fig. 1b). Since AG-1478 is a specific inhibitor of EGFR kinase (Moscatello DK 1998), AG-1478-induced inhibition of MBP synthesis indicated that EGFR signaling was required for Tβ4-mediated MBP synthesis. In addition to the pharmaceutical inhibitor- AG-1478, the endogenous cytoplasmic protein, Mig-6/ERRFI-1/Gene-33, specifically inhibits EGFR kinase (Zhang X 2007, Descot A 2009, Xu D 2006, Xu D 2005). To examine molecular mechanism of Tβ4-mediated EGFR signaling, we therefore investigated the expression of Mig-6 after Tβ4 treatment.
Tβ4 treatment down-regulates Mig-6 expression in rat SVZ progenitor cells and OPCs
To determine the effect of Tβ4 treatment on the expression of Mig-6, rat SVZ-neurospheres and OPCs were treated with Tβ4 at the dose of 0, 25, 50 and 100ng/ml for 7 days. The qrtPCR and Western blot revealed that Tβ4 treatment down-regulated Mig-6 expression in a dose dependent manner at mRNA and protein levels (Fig. 1c). To determine whether Mig-6 expression regulates EGFR phosphorylation and MBP expression, we transfected rat SVZ neurospheres and OPCs with Mig-6siRNA. The transfection efficacy was determined by qrtPCR (Fig. S1c). The Western blot data revealed that Mig-6siRNA transfection up-regulated both EGFR phosphorylation and MBP synthesis (Fig. 1c at bottom). These data indicated that Mig-6 expression was involved in MBP synthesis. The 3′-UTR of Mig-6 is a direct target of miR-200 in urothelial cancer cells (Adam L 2009). We therefore sought to investigate whether the 3′-UTR of Mig-6 is a direct target of miR-200 in rat SVZ-neurospheres and primary OPCs. The 3′-UTR of Mig-6 pmiR-GLO construct and the mutated/mismatched miR-200 target site in 3′-UTR of Mig-6 pmiR-GLO construct (mutant) were generated and analyzed based on luciferase assay in rat SVZ-neurospheres and primary OPCs according to manufacturer’s protocol (Promega Corporation, Madison, WI, USA), as described in Supplementary Methods. The transfection efficiencies for overexpression (>10 fold) and knock-down (>5 fold) of miR-200 family members were determined by qrtPCR (Fig. S2), and utilized for all the following experiments. The 3′-UTR of Mig-6 pmiR-GLO construct transfection markedly reduced luciferase activity which was significantly reversed after anti-miR-200a transfection or anti-miR-200b or anti-miR-200c. In contrast, mutated 3′-UTR of Mig-6 pmiR-GLO construct transfection had no effect on luciferase activity (Fig. S3). The co-transfection of Lenti-miR-200b-200a-429 or Lenti-miR-200c-141 with 3′-UTR of Mig-6 pmiR-GLO construct markedly augmented luciferase activities. In contrast, co-transfection of Lenti-miR-200b-200a-429 or Lenti-miR-200c-141 with mutated 3′-UTR of Mig-6 pmiR-GLO construct had no effect on luciferase activities (Fig. S3). These data indicate that 3′-UTR of Mig-6 is a direct target of miR-200a, −200b and −200c in rat SVZ-neurospheres and primary OPCs. We therefore investigated whether Tβ4 treatment affects the expression of the miR-200 family.
Tβ4 treatment induces expression of miR-200a in vitro in rat SVZ progenitor cells and embryonic primary OPCs, and in vivo in rats subjected to MCAO
To examine the effect of Tβ4 treatment on expression of miR-200 family members, rat SVZ-neurospheres and primary OPCs were treated with Tβ4 at the dose of 0, 25, 50 and 100ng/ml for 7 days. The qrtPCR data revealed that Tβ4 treatment markedly up-regulated miR-200a, miR-200b and miR-429 in vitro in a dose dependent manner (Fig. 1d), but rarely affected miR-200c and miR-141 (data not shown). These data indicate that Tβ4 treatment up-regulated miR-200b-a-429 cluster gene, but not miR-200c-141 cluster gene. These data were also consistent with in vivo up-regulation of miR-200a level in rats subjected to MCAO after Tβ4 (12mg/kg) treatment (Fig. 1e), as described in Supplementary Methods. In contrast, Tβ4 (12mg/kg) treatment had no effect on the expression level of miR-200b, −200c, −429 and −141 (data not shown). Tβ4 (12mg/kg) treatment, not only up-regulated miR-200a expression, but also down-regulated in vivo mRNA expression of miR-200 target Mig-6 (Fig. 1e bottom). However, MCAO had no effect on Mig-6 expression (Fig. 1e bottom). These data indicate that in vivo up-regulation of miR-200a level may activate EGFR signaling in vivo. However, the molecular mechanism how Tβ4 induces miR-200 expression is unknown. From genomic DNA of human chromosome-1 or mouse chromosome-4, a single tricistronic primary microRNA cluster gene encoding miR-200b, miR-200a and miR-429 (Fig. S4a) and, from genomic DNA of human chromosome-12 or mouse chromosome-6, another single bicistronic primary microRNA cluster gene encoding miR-200c and miR-141(Fig. S4b), are transcribed by RNA polymerase II (Trümbach D 2015). The miR-200b-a-429 cluster gene resides within an intergenic region that has an enhancer located approximately 5.1kb upstream of the miR-200b-a-429 transcriptional start site (Attema JL 2013). This enhancer that recruits sequence-specific transcription factors, and influences transcription of miR-200b-a-429 cluster gene, may be regulated by Tβ4 (Fig. S4a). In contrast, this enhancer may be missing in the promoter of primary miR-200b-a-429 cluster gene (Fig. S4b). However, further investigations are necessary to determine whether Tβ4 regulates directly transcription of mir-200b-a-429 cluster gene or indirectly regulates miRNA maturation on a post-transcriptional level.
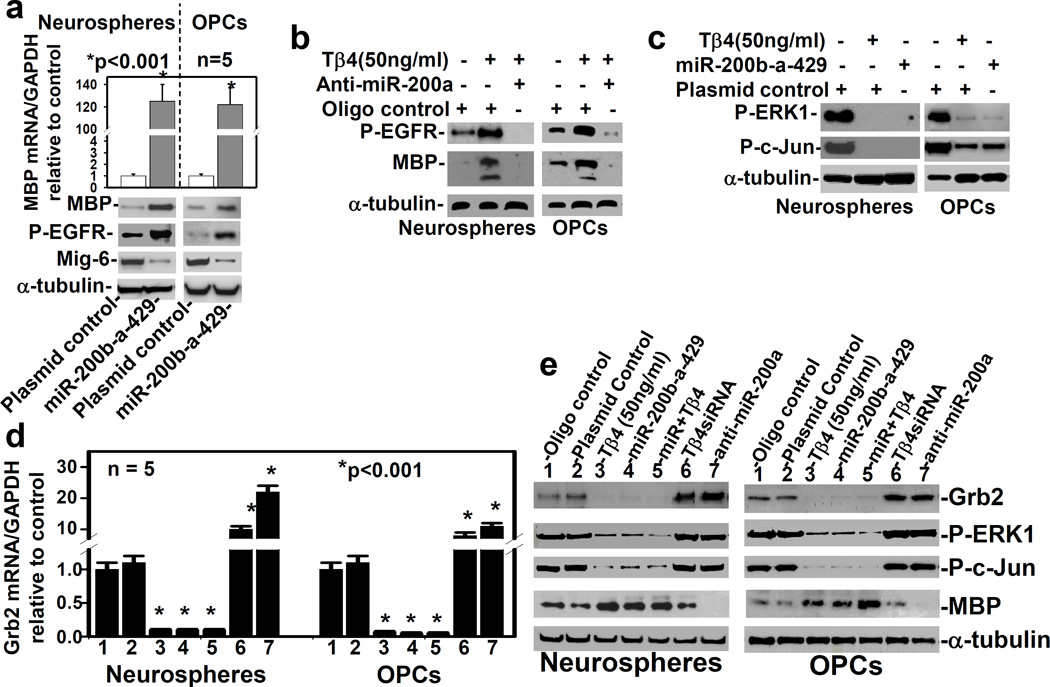
Transfection with miR-200b-a-429 cluster gene enhances EGFR signaling and MBP synthesis
As Tβ4 up-regulated miR-200a, b and 429, we hypothesized that miR-200 targets and reduces expression of Mig-6, a potent inhibitor of EGFR phosphorylation, and enhances EGFR signaling. To test this hypothesis, we transiently transfected rat SVZ-neurospheres, and primary OPCs, with one genomic cluster of miR-200 (e.g. miR-200b-200a-429) expression vector (pLenti4.1EX), in order to overexpress, or with anti-miR-200a to knock-down miR-200a, respectively. The qrtPCR and Western blot data demonstrated that the miR-200b-a-429 cluster transfection markedly up-regulated MBP at the mRNA and protein levels, along with decreasing Mig-6 protein level and increasing EGFR phosphorylation in rat SVZ neurospheres, and primary OPCs (Fig. 2a). To determine specificity of MBP antibody, whole blots were examined (Fig. S5). These data confirmed that MBP antibody strongly recognized MBP bands based on molecular size (∼15kDa) in rat SVZ neurospheres, and primary OPCs (Fig. S5). These data indicated that miR-200b-a-429 cluster transfection targeted Mig-6, increased EGFR phosphorylation and induced MBP synthesis and oligodendrocyte differentiation.
Fig. 2. Transfection with miR-200b-a-429 cluster enhances EGFR signaling and MBP synthesis, and inhibits Grb2 synthesis and ERK1phosphorylation.
a) The qrtPCR and Western blot analysis, after transfection with plasmid control, and miR-200b-a-429 cluster expression vector (miR-200b-a-429); b) Western blot analysis after treatment with/without Tβ4 and transfection with scrambled oligo control (oligo control), and with anti-miR-200a; c) Western blot analysis of plasmid control transfected cells followed by treatment with/without Tβ4, and miR-200b-a-429 cluster transfected cells; and, the qrtPCR (d) and Western blot (e) analyses in 7 samples (shown at the top of the gel) were performed in the adult rat SVZ neurospheres/progenitor cells (Neurospheres) and primary rat embryonic OPCs (OPCs) in the five independent experiments (n=5). Loading of the samples for all Western blot analyses is normalized with α-tubulin. The p-value <0.05 was considered as significantly different.
To determine whether expression of miR-200a was required for Tβ4-mediated MBP synthesis, we transfected Tβ4 (50ng/ml) treated cells (SVZ-neurospheres and OPCs) with anti-miR-200a. Western blot analysis showed that anti-miR-200a transfection reversed the Tβ4 effect on EGFR phosphorylation and MBP synthesis (Fig. 2b). These data indicated that Tβ4 induced MBP synthesis in an miR-200a dependent EGFR signaling pathway. However, enhanced EGFR signaling usually activates or phosphorylates ERK1 leading to activation/phoshorylation of a transcription factor- c-Jun, a potent inhibitor of MBP promoter (Santra M 2014, Santra M 2012, Daub H 1996). We therefore investigated whether miR-200b-a-429 cluster transfection induces phosphorylation of ERK1 in rat SVZ-neurospheres, and primary OPCs. Western blot data revealed that miR-200b-a-429 cluster transfection surprisingly down-regulated phosphorylation of ERK1 and c-Jun (Fig. 2c). Tβ4 (50ng/ml) treatment also consistently suppressed phosphorylation of ERK1 and c-Jun (Fig. 2c), as previously reported (Santra M 2012, Santra M 2014). These data suggested that miR-200a may target any of the adaptors or mediators of EGFR signaling. We therefore investigated TargetScan database (http://www.mirbase.org) of miR-200a for the adaptor and mediators, which drive EGFR/Grb2/ERK signaling pathway.
Transfection with miR-200b-a-429 cluster inhibits phosphorylation of ERK1 after targeting Grb2, a key adaptor for EGFR/Grb2/Ras/ERK1 signaling pathway
Based on TargetScan database (http://www.mirbase.org), we found that 3′-UTR of Grb2 was direct target of miR200a. The direct target of miR200a for Grb2 has been previously confirmed in HEK293T cells (Liu Y 2013). The rat SVZ neurospheres and primary OPCs were therefore transfected with the miR-200b-200a-429 cluster, and treated with Tβ4 treatment (50ng/ml). The qrtPCR and Western blot analysis revealed that transfection and treatment alone and, combined transfection with treatment markedly down regulated Grb2 expression at the mRNA and protein levels, and inhibited phosphorylation of ERK1 and c-Jun, but up-regulated MBP (Fig. 2d, e). In contrast, Tβ4siRNA or anti-miR-200a transfection reversed the effect of miR-200b-200a-429 cluster transfection, and Tβ4 treatment (50ng/ml) on Grb2 expression, phosphorylation of ERK1 and c-Jun, and MBP expression (Fig. 2d, e). These data are also consistent with the previous data of miR-200a transfection which targets Grb2 in mouse embryonic stem cells (Liu Y 2013). These data are also consistent with our previous results on induction of OPC differentiation into oligodendrocyte after reduction of phosphorylation of ERK1 and c-Jun (Santra M 2014, Santra M 2012). Inhibition of ERK1 signaling enhances the EGFR/PI3K/AKT signaling pathway (Gan Y1 2010). We therefore investigated the effect of Tβ4 on AKT activation in the EGFR/PI3K/AKT signaling pathway.
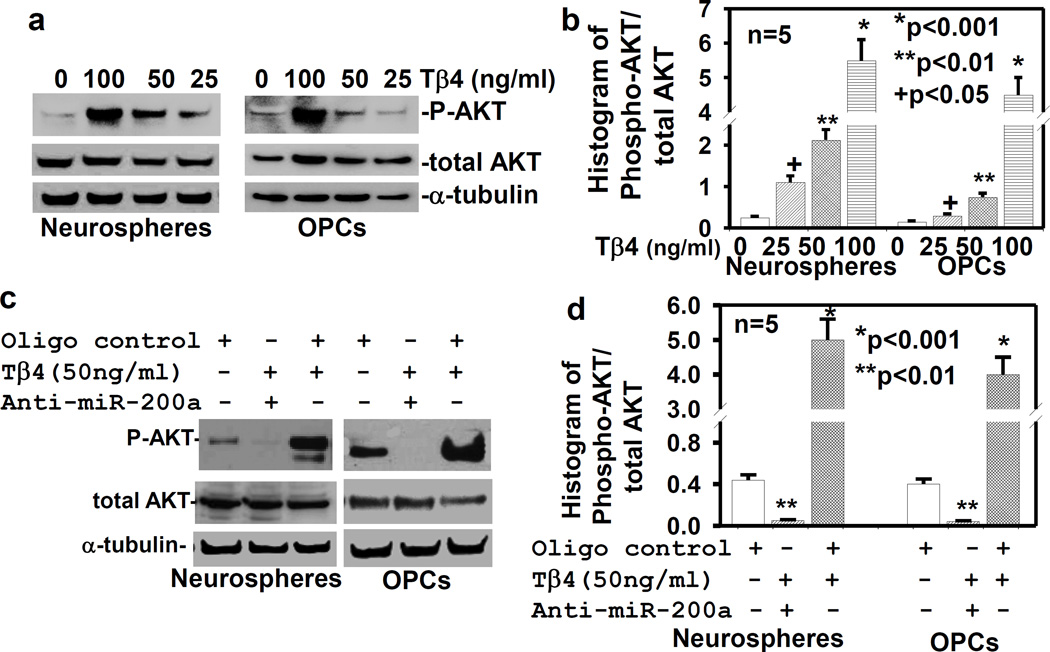
Tβ4 treatment activates AKT in miR-200a dependent pathway
To determine the effect of Tβ4 treatment on AKT activation, the rat SVZ-neurospheres and primary OPCs were treated with Tβ4 at the dose of 0, 25, 50 and 100ng/ml for 7 days. Western blot data demonstrated that Tβ4 treatment induced activation/phosphorylation of AKT in a dose dependent manner (Fig. 3a, b). In contrast, anti-miR-200a transfection reversed the Tβ4 effect on AKT activation (Fig. 3c, d). These data indicated that Tβ4 treatment induced AKT activation in miR-200a dependent pathway, and suggested that miR-200a may target endogenous PI3K/AKT inhibitors. Two miR-200a targets, FOG-2 and PTEN negatively regulate PI3K/AKT pathway (Hyun S 2009, Yang Y 2014, Becker LE 2015, Li H 2014). We therefore examined the effect of miR-200a on the expression of FOG-2 and PTEN, and AKT activation in the rat SVZ-neurospheres and primary OPCs.
Fig. 3. Tβ4 treatment induces activation/phosphorylation of AKT.
a) Western blot analysis after the treatment with Tβ4 at different doses for AKT and AKT phosphorylation (P-AKT); b) Quantitative histogram analysis of the AKT phosphorylation/activation from Western blot (a) data; c) Western blot analysis after transfection with scrambled oligo control (oligo control) followed by treatment with/without Tβ4, and anti-miR-200a; d) Quantitative histogram analysis of the AKT phosphorylation/activation from Western blot (c) data, were performed in the adult rat SVZ neurospheres/progenitor cells (Neurospheres) and primary rat embryonic OPCs (OPCs) in the five independent experiments (n=5). Loading of the samples was normalized with α-tubulin. The p-value<0.05, was considered, as significance difference.
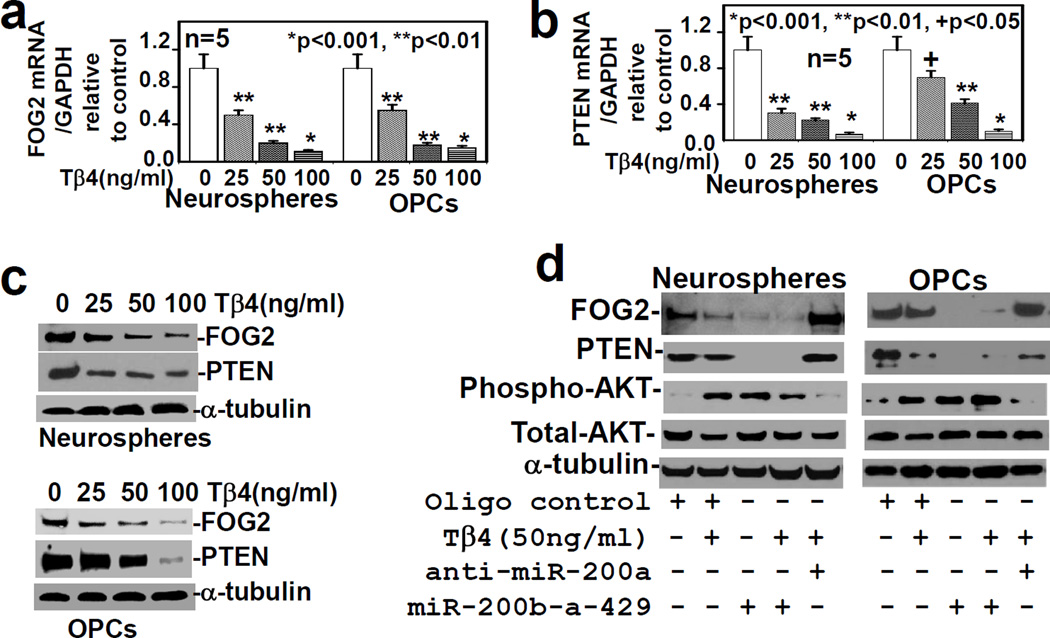
Tβ4 treatment and transfection with miR-200b-a-429 cluster down-regulate PI3K inhibitors- FOG2 and PTEN, and activates AKT
To determine the effect of Tβ4 treatment on the expression of FOG-2 and PTEN, rat SVZ-neurospheres and primary OPCs were treated with Tβ4 at the dose of 0, 25, 50 and 100ng/ml for 7 days. QrtPCR and Western blot data demonstrated that expression of FOG-2 and PTEN was down-regulated in a dose dependent manner in mRNA and protein levels (Fig. 4a, b, c). To examine the effect of miR-200a on the expression of FOG-2 and PTEN, and AKT phosphorylation/activation, the rat SVZ-neurospheres and primary OPCs were transfected with miR-200b-a-429 cluster. Western blot data demonstrated that miR-200b-a-429 cluster transfection, Tβ4 treatment (50ng/ml), and miR-200b-a-429 cluster transfection combined with Tβ4 treatment (50ng/ml) down-regulated the expression of FOG2 and PTEN, but enhanced AKT phosphorylation/activation in the rat SVZ-neurospheres and primary OPCs (Fig. 4d). In contrast, anti-miR-200a transfection reversed the effect of Tβ4 treatment on expression of FOG-2 and PTEN, and phosphorylation/activation of AKT in the rat SVZ-neurospheres and primary OPCs (Fig. 4d). These data demonstrated that Tβ4 treatment increased AKT activation in the rat SVZ neurospheres and primary OPCs after miR-200a–mediated inhibition of expression of FOG-2 and PTEN. Enhanced AKT activation is involved in cell survival and also enhances MDM2-mediated ubiquitination and degradation of p53, an inducer of pro-apoptotic genes (Zhou BP 2001). The miR-200a directly targets p53 which is up-regulated after ischemic stroke in experimental animal models and triggers cell death, as previously reported (Becker LE1 2012, Hong LZ 2010, Chopp M 1992). Moreover, ischemic lesion is significantly reduced in p53 knockout mice, subjected to MCAO (Crumrine RC 1994). We therefore sought to investigate, whether Tβ4 treatment or miR-200b-a-429 cluster transfection affects p53 expression in the rat SVZ-neurospheres and primary OPCs, and protect these cells from ischemic insult such as OGD.
Fig. 4. Transfection with miR-200b-a-429 cluster reduces PI3K inhibitors- FOG2 and PTEN, and increases AKT activation.
The qrtPCR analysis for FOG-2 (a) and PTEN (b), and Western blot (c) after the treatment with Tβ4 at different doses; and d) Western blot analysis after transfection with scrambled oligo control (Oligo control), anti-miR-200a and miR-200b-a-429 cluster followed by treatment with/without Tβ4, were performed in the adult rat SVZ neurospheres/progenitor cells (Neurospheres) and primary rat embryonic OPCs (OPCs) in five independent experiments (n=5). Loading of the samples was normalized with α-tubulin. The p-value <0.05, was considered as significantly different.
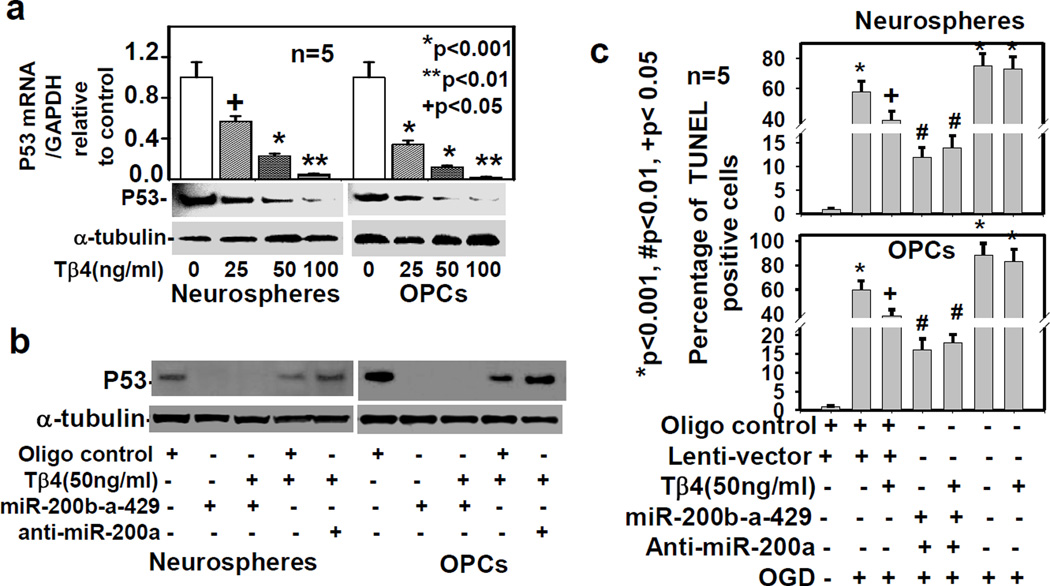
Tβ4 treatment and miR-200b-a-429 cluster transfection down-regulate p53 expression
To examine the effect of Tβ4 treatment on p53 expression, the rat SVZ neurospheres and primary OPCs were treated with Tβ4 at the dose of 0, 25, 50 and 100ng/ml for 7 days. Western blot and qrtPCR data demonstrated that p53 expression were down-regulated in a dose dependent manner at the mRNA and protein levels (Fig. 5a). To examine the effect of miR-200a on p53 expression, we transfected the rat SVZ-neurospheres and primary OPCs, with miR-200b-a-429 cluster and anti-miR-200a. Western blot data showed marked p53 down-regulation after Tβ4 (50ng/ml) treatment, miR-200b-a-429 cluster transfection, and combined transfection with Tβ4 treatment (Fig. 5b). In contrast, anti-miR-200a transfection reversed the inhibitory effect of Tβ4 treatment on expression of p53 in the rat SVZ neurospheres and primary OPCs (Fig. 5b). Transfection with miR-200b-a-429 cluster reduced p53 mRNA at 0.5±0.06 fold, based on qrtPCR analysis. In contrast, transfection with miR-200b-a-429 markedly reduced p53 protein at 110±10.55 fold based on histogram analysis of Western blot in 5 independent experiments. These data indicate that miR-200b-a-429 cluster transfection down-regulated more p53 protein than p53 mRNA. The miR-200a not only directly targets p53, but also induces degradation of p53 protein via AKT signaling pathway. Since Tβ4 treatment and miR-200b-a-429 transfection reduced p53 expression, we therefore examine the effect of Tβ4 treatment and miR-200b-a-429 transfection on OGD-mediated apoptosis by TUNEL staining.
Fig. 5. Transfection with miR-200b-a-429 cluster down-regulates p53 and inhibits OGD-induced apoptosis.
The p53 expression was analyzed by qrtPCR and Western blot after the treatment with Tβ4 at different doses (a); and by Western blot (c) after transfection with scrambled oligo control (Oligo control), anti-miR-200a and miR-200b-a-429 cluster followed by treatment with/without Tβ4 (b) in the adult rat SVZ neurospheres/progenitor cells (Neurospheres) and primary rat embryonic OPCs (OPCs) in five independent experiments (n=5). Loading of the samples was normalized with α-tubulin. Apoptosis (c) was quantified in percentage of TUNEL positive in the adult rat SVZ-neurospheres (Neurospheres) and rat embryonic primary OPCs (OPCs) after Tβ4 treatment and different transfections as shown at bottom. The asterisk (*) indicates significant induction of OGD-mediated TUNEL positive cells. In contrast, the number (#) and plus (+) indicate significant reduction of OGD-mediated TUNEL positive cells after Tβ4 treatment or miR-200 clusters transfection in five independent experiments (n=5). The p-value<0.05, was considered, as significance difference.
Tβ4 treatment or miR-200b-a-429 cluster transfection inhibits OGD-mediated apoptosis
To determine the effect of Tβ4 treatment and miR-200b-a-429 transfection on OGD-mediated apoptosis, the rat SVZ neurospheres and primary OPCs were treated with Tβ4 (50ng/ml) for 4 days or transfected with miR-200b-a-429 cluster. On day 2, these cells were exposed to OGD for 4h followed by TUNEL staining on day 4. TUNEL staining data indicated that Tβ4 treatment or miR-200b-a-429 transfection markedly reduced TUNEL positive cells in compared to control cells subjected to OGD for 4h (Fig. 5c, Figs. S6, S7). In contrast, anti-miR-200a transfection reversed the anti-apoptotic effect of Tβ4 in the rat SVZ neurospheres and primary OPCs (Fig. 5c, Figs. S6, S7). These data indicated that Tβ4 treatment protected SVZ progenitor cells and OPCs from ischemic insult, OGD. These data were also consistent with neuroprotective effect of miR-200 transfection in NPC line, Neuro-2a, subjected to OGD for 16h (Lee ST 2010), and suggest Tβ4 and miR-200 family contribute to the therapeutic benefits observed for treatment of neurologic diseases e.g. stroke and traumatic brain injury (Lee ST 2010, Morris DC 2010a, Xiong Y 2010). As miR-200 family shows similarity in their RNA sequences, we examined whether overexpression of mir-200c-141 cluster exhibits the similar effects as the miR-200b-a-429 cluster. Although Tβ4 treatment had no effect on regulation of miR-200c-141 cluster, transfection of miR-200c-141 cluster showed the same effects as miR-200b-a-429 cluster on inhibition of OGD-induced apoptosis (Figs. S6, S7); down-regulation of GRB2, Mig-6, Fog-2, Pten and P53; and upregulation of MBP and phospho-AKT (Fig. S8a, b). These data suggest the therapeutic benefit of the entire miR-200 family for treatment of neurologic diseases e.g. stroke and traumatic brain injury. We therefore investigated whether Tβ4 treatment down-regulates miR-200 targets in vivo in rats subjected to MCAO.
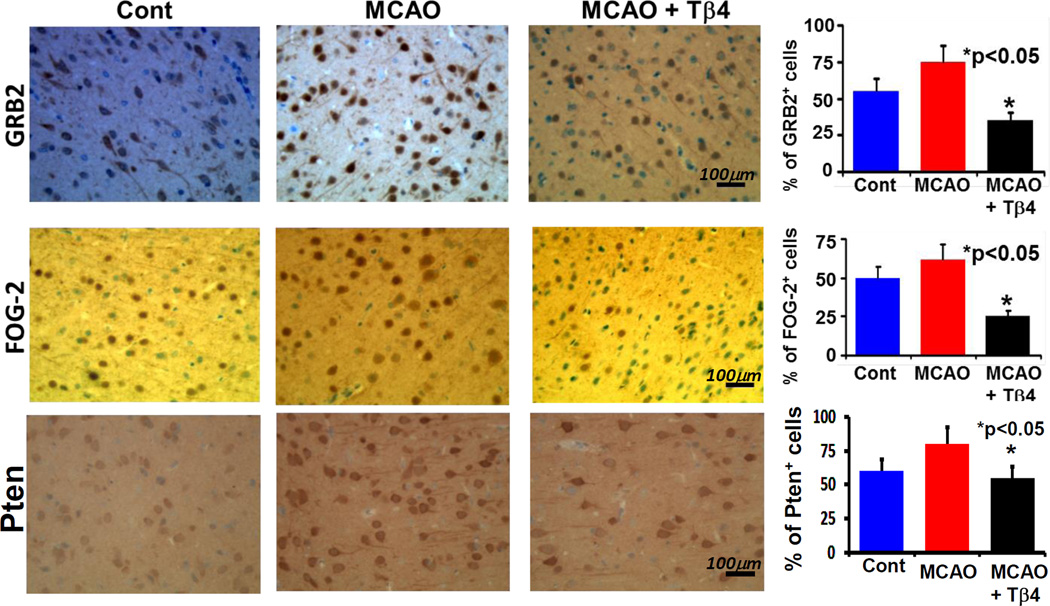
Effect of in vivo Tβ4 treatment on miR-200 targets e.g. GRB2, FOG-2 and Pten in rats subjected to MCAO
To examine miR-200 targets in vivo, paraffin embedded brain slides from normal rats, rats subjected to MCAO and rats subjected to MCAO followed by treatment with Tβ4 (12mg/kg), were immunostained with DAB for GRB2, FOG-2 and Pten antibodies analyzed in peri-infarct area of rat brain subjected to MCAO. In normal rat brain, tissue from the area corresponding to the peri-infarct area of rat brain subjected to MCAO was selected for analysis. These data showed significant down-regulation of miR-200a targets- e.g. GRB2, FOG-2 and Pten (Fig. 6). These in vivo data were consistent with in vitro down-regulation of GRB2, FOG-2 and Pten. Thus, Tβ4 treatment in vivo and in vitro may induce AKT activation and protect brain cells from MCAO-mediated apoptosis. Our data and observations of miR-200-mediated neuroprotection (Lee ST 2010), Tβ4-mediated improvement of functional neurological outcome in a rat model of embolic stroke(Morris DC 2010a), and neuroprotection in a rat model of traumatic brain injury(Xiong Y 2012b) indicate therapeutic importance of Tβ4 and miR-200 for the treatment of stroke and traumatic brain injury.
Fig. 6. Immunohistochemistry of miR-200 targets e.g. GRB2, FOG-2 and Pten in vivo in rats subjected to MCAO after Tβ4 treatment.
Paraffin embedded brain slides from normal rats (normal), rats subjected to MCAO (MCAO) and rats subjected to MCAO followed by treatment with 12mg/kg Tβ4 (MCAO+Tβ4), were immunostained with DAB for GRB2, FOG-2 and Pten antibodies. Location of images was in the peri-infarct areas. Bar graphs at right indicate quantitative analysis of corresponding antibodies (e.g. GRB2, FOG-2 and Pten) positive (+) cells in percentage (%).
Discussion
We demonstrated that Tβ4 up-regulated miR-200a, −200b and −429 which belong to miR-200b-a-429 cluster gene. Tβ4 treatment down-regulated important regulators of EGFR signaling, which are known targets of miR-200a, in favor of oligodendrogenesis, and protection against OGD in a cell culture model. Up-regulation of miR-200a (in vivo and in vitro), and miR-200b and −429 (in vitro) after Tβ4 treatment, down-regulated miR-200a target, Grb2, a key adapter of EGFR/Grb2/ERK1 signaling pathway, leading to inhibition of phosphorylation/activation of a transcription factor, c-Jun. Activation of c-Jun negatively regulates the MBP promoter and MBP synthesis, as previously reported (Santra M 2012, Santra M 2014, Chew LJ 2010). In current study, we demonstrated that Tβ4 induced MBP expression in a novel miR-200a regulated molecular mechanism of inhibition of EGFR/Grb2/ERK1/c-Jun signaling. When the EGFR/Grb2/ERK1 signaling is blocked, EGFR/PI3K/AKT signaling is usually activated (Gan Y1 2010). Our data demonstrated that Tβ4 treatment markedly up-regulated AKT activation/phosphorylation leading to cell survival in a novel miR-200a dependent pathway. Tβ4 treatment (in vitro and in vivo) or miR-200b-a-429 cluster transfection (in vitro) down-regulated the known targets of miR-200a e.g. the potent EGFR kinase inhibitor-ERRFI-1/Mig-6 and inhibitors of PI3K/AKT signaling- FOG2 and PTEN, leading to markedly activation of AKT. ERRFI-1/Mig-6 is a cytosolic endogenous protein which binds and inhibits phosphorylation/activation of EGFR (Zhang X 2007, Descot A 2009, Xu D 2006, Xu D 2005). After miR-200-mediated down-regulation of Mig-6, EGFR was constitutively activated leading to PI3K/AKT activation. AKT was further activated, when PI3K inhibitors, FOG2 and PTEN were down-regulated after Tβ4 treatment or miR-200b-a-429 cluster transfection. As anti-miR-200a transfection reversed the Tβ4 effect on AKT activation, Tβ4 treatment markedly activated AKT via a novel miR-200 dependent EGFR/PI3K/AKT pathway. Tβ4 treatment or miR-200b-a-429 cluster transfection down-regulated in vitro another known target of miR-200a, p53, an inducer of pro-apoptotic genes, and inhibited ischemic-induced cell death in a cell culture model of OGD. Since anti-miR-200a transfection reversed the Tβ4 effect on p53 expression and OGD-mediated apoptosis, Tβ4 treatment markedly activated AKT as well as reduced p53 expression leading to inhibition of apoptosis via miR-200a dependent EGFR/PI3K/AKT signaling pathway. These data were also consistent with neuroprotective effect of miR-200 transfection in NPC line, Neuro-2a, subjected to OGD for 16h (Lee ST 2010).
EGFR controls cell growth, differentiation, migration and oligodendrogenesis in mammalian brain (Aguirre A 2007a, Aguirre A 2007b). When EGFR’s intrinsic kinase is activated, it drives several downstream intracellular signaling pathways, e.g. Grb2/Ras/MEK/ERK and PI3K/AKT (Hayakawa-Yano Y1 2007, Gan Y1 2010). Our data demonstrated that Tβ4-mediated up-regulation of miR-200 family members down-regulated Grb2, blocked EGFR/Grb2/Ras/MEK/ERK/cJun signaling, but activated EGFR/PI3K/AKT signaling. The reduced EGFR signaling attenuates MBP expression and oligodendrogenesis in the wa2 mouse (Aguirre A 2007b, Aguirre A 2007a). EGFR/PI3K/AKT signaling may be required for MBP expression. EGFR/Grb2/Ras/MEK/ERK signaling induces proliferation. In contrast, EGFR/PI3K/AKT signaling pathway mediates cell survival/differentiation (Furnari FB 2007). EGFR/PI3K/AKT signaling is required, but EGFR/Grb2/Ras/MEK/ERK is not required for oligodendrogenesis in the number of Olig2+ progenitor cells in the developing spinal cord (Hayakawa-Yano Y1 2007). When cell differentiation switches on, the proliferation of cells therefore turns off. In the current study, our data also demonstrated that Tβ4-induced miR-200 activated EGFR/PI3K/AKT signaling which was required for MBP synthesis.
AKT is a serine/threonine kinase which phosphorylates more than 30 downstream targets/substrates and regulates cell survival, growth, differentiation, migration, and metabolism in a cell-specific manner (Somanath PR 2006). In OPCs, activation of AKT induces cell survival, MBP synthesis and differentiation into myelinating mature oligodendrocytes in vivo in transgenic (Plp-Akt-DD) mice (Frederick TJ 2007, Yang W1 2007, Flores AI 2000, Pang Y 2007, Narayanan SP 2009, Flores AI 2008). In the current study, we demonstrated that, Tβ4-induced miR-200, down-regulated FOG2 and PTEN, which were the endogenous potent inhibitors of PI3K. As a result, AKT was activated. FOG2 inhibits active PI3K complex formation between p85 and p110 by forming inactivation PI3K complex between p85 and FOG-2 (Hyun S 2009). Thus, Tβ4- and miR-200-induced down-regulation of FOG2, enhanced active PI3K complex (p110 and p85a) formation, PI3K activation and AKT activation (Hyun S 2009). PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3) which phosphorylates/activates AKT (Sansal I 2004, Mellinghoff IK 2005), whereas PTEN deposphorylates PIP3 to PIP2 by its lipid phosphatase activity and inhibits PI3K/AKT activation (Sansal I 2004, Mellinghoff IK 2005). Tβ4- and miR-200-induced down-regulation of PTEN, therefore, induced AKT activation. The rationale for choosing FOG2 and PTEN is to determine cell survival signaling pathway via PI3K/AKT activation. FOG2 and PTEN both negatively regulate PI3K/AKT activation. Down-regulation of both of these potent endogenous inhibitors of PI3K/AKT results in strong AKT activation leading to cell survival and protection of cells from OGD-mediated apoptosis.
The activation of AKT has been shown to phosphorylate MDM2, and increase the nuclear localization of MDM2. Phosphorylated MDM2 interacts with p300 and p53, which undergoes ubiquitination, and degradation of p53 (Zhou BP 2001). The p53 is a transcription factor which induces the pro-apoptotic genes e.g. Noxa and PUMA in many cells, specially neurons (Cregan SP 2004). PUMA is localized in mitochondria where it interacts with Bcl-2, Bcl-XL and Bax. As a result, cytochrome c is released, thereby caspases-9 and −3 are activated, and the cells including neurons and OPCs, undergo apoptosis (Cregan SP 2004, Pang Y 2007). Our present data demonstrated that Tβ4 treatment down-regulated p53 via miR-200a–mediated direct effect and via AKT activation, and inhibited OGD-mediated apoptosis. Ischemic stroke induces expression of p53 which causes neuronal death in an animal model of embolic stroke, as previously reported (Chopp M 1992). Other neuronal death paradigms e.g. hypoxia and excitotoxicity have been shown to up-regulate the expression of p53 (Banasiak KJ1 1998, Halterman MW 1999). Postmitotic neurons undergo apoptosis after overexpression of p53 (Slack RS1 1996 Cregan SP 1999). In contrast, excitotoxicity and stroke result in reduced brain damage in p53 null mice in compared to wild-type mice (Crumrine RC 1994, Morrison RS1 1996). Thus, Tβ4- or miR-200b-a-429 cluster--mediated AKT activation and p53 down-regulation may be involved in inhibition of OGD-induced apoptosis and may provide a new therapeutic approach and implication for the treatment of neurological injury. In addition, the reduction of OGD-induced apoptosis by Tβ4- or miR-200b-a-429 cluster suggest that Tβ4 treatment or miR-200b-a-429 cluster transfection may also enhance the survival of stroke-induced newborn cells (OPCs and NPCs) in the SVZ, and thereby promote neurological recovery (Arvidsson A 2002, Zhang R 2004a, Zhang R 2004b, Parent JM 2002, Jin K 2001, Jin K 2006, Zhang RL 2001b, Macas J 2006, Minger SL 2007, Curtis MA 2011, Zhang ZG 2009, Zhang R 2013). These newborn NPCs migrate from the SVZ to the infarct area of ischemic stroke. These migrated NPCs differentiate into parenchymal cells including neurons. However, in the striatum, only 20% of the newly generated cells survive, resulting in an estimated replacement of only 0.2% of the cells that are lost as a result of ischemia in a rat model of embolic stroke (Arvidsson A 2002). Our data are consistent with observations that Tβ4 and miR-200 (Lee ST 2010) provide significant therapeutic benefits for the treatment of stroke and traumatic brain injury (Morris DC 2010a, Xiong Y 2012a).
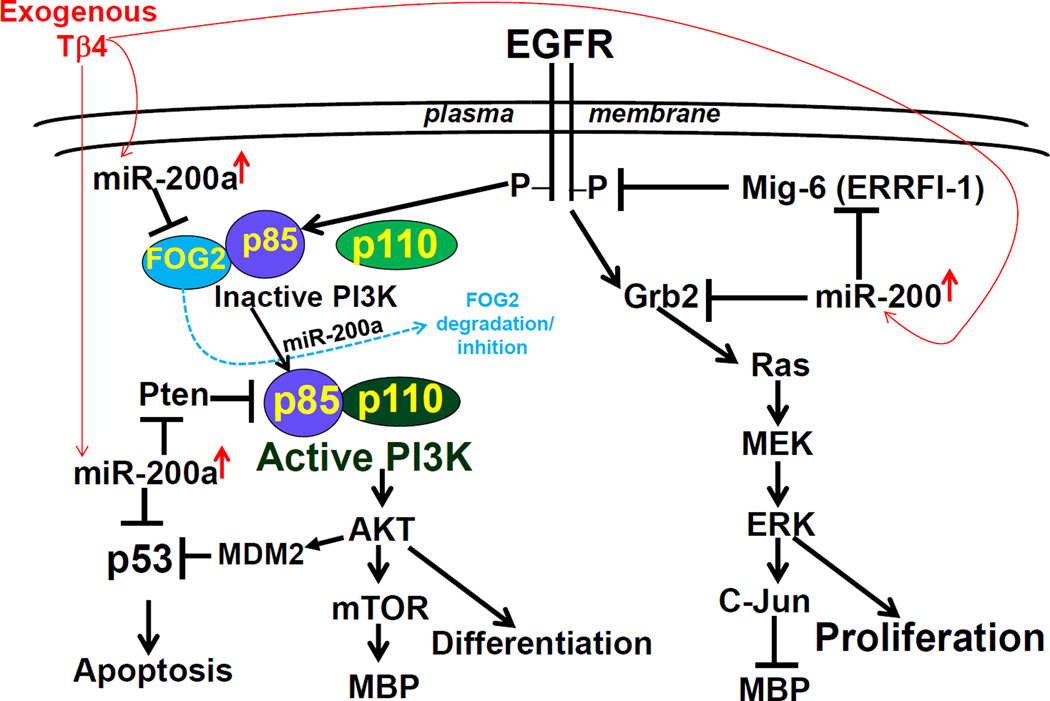
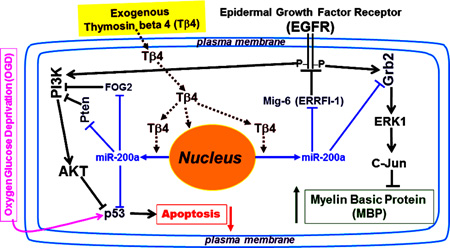
In summary, Tβ4 treatment up-regulated miR-200 expression which targeted Grb2 and blocked EGFR/Grb2/Ras/MEK/ERK/c-Jun signaling pathway to proliferation (Fig. 7). Blockage of proliferation signaling induced MBP synthesis and OPC differentiation, as c-Jun activation negatively regulate MBP synthesis. Tβ4-induced miR-200 down-regulated EGFR inhibitor- ERRFI-1/Mig6, and PI3K inhibitors (FOG2 and PTEN), as a result, AKT was activated (Fig. 7). The enhanced AKT activation also induces MBP via EGFR/PI3K/AKT/mTOR signaling pathway and OPC differentiation, as previously reported (Narayanan SP 2009, Flores AI 2008). The enhanced AKT activation phosphorylates MDM2 which induces p53-ubiquitination and degradation (Fig. 7). Tβ4-induced miR-200a, also directly down-regulated p53 expression (Fig. 7). Since p53 induces apoptosis in ischemic cells, rat SVZ progenitor cells and embryonic primary OPCs may be protected from OGD-mediated apoptosis (Fig. 7). These data suggest that Tβ4 via miR-200 may contribute the therapeutic benefits for stroke and other neuronal diseases associated with demyelination disorders (Scafidi J 2014, Baumann N 2001).
Fig. 7. A summarizing schematic diagram of mechanistic cascades for Tβ4-induced OPC differentiation and inhibition of OGD-mediated apoptosis in novel miR-200-regulated EGFR signaling pathways.
Exogenous Tβ4 administration up-regulated miR-200a which targeted Grb2, blocked proliferating signal (EGFR/Grb2/ERK1), and thereby induced OPC differentiation by increasing MBP (a marker of OPC differentiation) synthesis. Tβ4-induced up-regulation of miR-200a targeted endogenous inhibitors of EGFR signaling, e.g. Mig-6 for EGFR and, FOG-2 and Pten for PI3K. As a result, AKT was markedly activated. AKT activation usually induces 1) cell survival, 2) OPC differentiation via AKT/mTOR/MBP, and 3) inhibition of apoptosis via MDM2-mediated p53 degradation. Tβ4-induced miR-200a directly targeted P53, reduced p53 synthesis and inhibited P53-mediated apoptosis. Protection of progenitor cells from OGD-mediated apoptosis may contribute the therapeutic benefits of Tβ4 and miR-200 for stroke and other neuronal diseases associated with demyelination disorders.
Supplementary Material
Acknowledgements
We thank RegeneRx Biopharmaceuticals Inc, Rockville, MD for providing us Tβ4. A patent (PCT/US10/28839) has been granted on Sept 22, 2011 for use of Tβ4 in Improving Neurological Outcome after Neural Injury and Neurodegenerative Disease. This work has been supported by NIH, R01 AG038648 (DM), R01 NS075156 (ZG) and R01 NS088656 (MC).
Abbreviations
- Tβ4
Thymosin beta 4
- NPC
neuro-progenitor cell
- OPC
oligoprogenitor cells
- MBP
myelin basic protein
- EGFR
Epidermal growth factor receptor
- Grb2
growth factor receptor-bound protein 2
- FOG2
Friend of GATA 2
- PTEN
Phosphatase and tensin homolog deleted in chromosome 10
- PI3K
phosphatidylinositol-3-kinase
- OGD
oxygen glucose deprivation
- 3’UTR
3’untranslated region
References
- Adam LZM, Choi W, Qi W, Nicoloso M, Arora A, Calin G, Wang H, Siefker-Radtke A, McConkey D, Bar-Eli M, Dinney C. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15:5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. PMID: 19671845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre ADJ, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007a;10:990–1002. doi: 10.1038/nn1938. PMID: 1761827. [DOI] [PubMed] [Google Scholar]
- Aguirre AGV. Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination. Neuron Glia Biol. 2007b;3:209–220. doi: 10.1017/S1740925X08000082. PMID: 18634612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AGV. Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination. Neuron Glia Biol. 2007c;3:209–220. doi: 10.1017/S1740925X08000082. PMID: 18634612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre ARM, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. PMID: 20844536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre ART, Ratner N, Gallo V. Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors. J Neurosci. 2005;25:11092–11106. doi: 10.1523/JNEUROSCI.2981-05.2005. PMID: 16319309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson ACT, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. PMID: 12161747. [DOI] [PubMed] [Google Scholar]
- Attema JLBA, Lim YY, Kolesnikoff N, Lawrence DM, Pillman KA, Smith E, Drew PA, Khew-Goodall Y, Shannon F, Goodall GJ. Identification of an enhancer that increases miR-200b∼200a∼429 gene expression in breast cancer cells. PLoS One. 2013;8:e75517. doi: 10.1371/journal.pone.0075517. PMID: 24086551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasiak KJ1HG. Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res. 1998;797:295–304. doi: 10.1016/s0006-8993(98)00286-8. PMID: 9666152. [DOI] [PubMed] [Google Scholar]
- Baumann NP-DD. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. PMID: 11274346. [DOI] [PubMed] [Google Scholar]
- Becker LE1LZ, Chen W, Xiong W, Kong M, Li Y. A systematic screen reveals MicroRNA clusters that significantly regulate four major signaling pathways. PLoS One. 2012;7:e48474. doi: 10.1371/journal.pone.0048474. PMID: 23144891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker LETA, Lu Z, Li Y. The role of miR-200a in mammalian epithelial cell transformation. Carcinogenesis. 2015 Jan;36(1):2–12. doi: 10.1093/carcin/bgu202. 36, 2–12 PMID: 25239643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YBV, Peng J, Hurlock EC, Tallquist M, Li J, Lu QR. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007;2:1044–1051. doi: 10.1038/nprot.2007.149. PMID: 17546009. [DOI] [PubMed] [Google Scholar]
- Chew LJCW, Cheng Y, Gallo V. Mechanisms of regulation of oligodendrocyte development by p38 mitogen-activated protein kinase. J Neurosci. 2010;30:11011–11027. doi: 10.1523/JNEUROSCI.2546-10.2010. PMID: 20720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi PSZL, Choi WY, Caron S, Alvarez-Saavedra E, Miska EA, McManus M, Harfe B, Giraldez AJ, Horvitz HR, Schier AF, Dulac C. Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron. 2008;57:41–55. doi: 10.1016/j.neuron.2007.11.018. PMID: 18184563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp MLY, Zhang ZG, Freytag SO. p53 expression in brain after middle cerebral artery occlusion in the rat. Biochem Biophys Res Commun. 1992;182:1201–1207. doi: 10.1016/0006-291x(92)91859-o. PMID: 1540165. [DOI] [PubMed] [Google Scholar]
- Cregan SPAN, Maclaurin JG, Callaghan SM, Fortin A, Cheung EC, Guberman DS, Park DS, Slack RS. p53 activation domain 1 is essential for PUMA upregulation and p53-mediated neuronal cell death. J Neurosci. 2004;24:10003–10012. doi: 10.1523/JNEUROSCI.2114-04.2004. PMID: 15525786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregan SPMJ, Craig CG, Robertson GS, Nicholson DW, Park DS, Slack RS. Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J Neurosci. 1999;19:7860–7869. doi: 10.1523/JNEUROSCI.19-18-07860.1999. PMID: 10479688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumrine RCTA, Morgan PF. Attenuation of p53 expression protects against focal ischemic damage in transgenic mice. J Cereb Blood Flow Metab. 1994;14:887–891. doi: 10.1038/jcbfm.1994.119. PMID: 7929653. [DOI] [PubMed] [Google Scholar]
- Curtis MAKM, Faull RL. Neurogenesis in humans. Eur J Neurosci. 2011;33:1170–1174. doi: 10.1111/j.1460-9568.2011.07616.x. PMID: 21395861. [DOI] [PubMed] [Google Scholar]
- Daub HWF, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. PMID: 8596637. [DOI] [PubMed] [Google Scholar]
- Descot AHR, Shaposhnikov D, Reschke M, Ullrich A, Posern G. Negative regulation of the EGFR-MAPK cascade by actin-MAL-mediated Mig6/Errfi-1 induction. Mol Cell. 2009;35:291–304. doi: 10.1016/j.molcel.2009.07.015. PMID: 19683494. [DOI] [PubMed] [Google Scholar]
- Doetsch FPL, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. PMID:12495619. [DOI] [PubMed] [Google Scholar]
- Flores AIMB, Matsui T, Ogawa W, Rosenzweig A, Okamoto T, Macklin WB. Akt-mediated survival of oligodendrocytes induced by neuregulins. J Neurosci. 2000;20:7622–7630. doi: 10.1523/JNEUROSCI.20-20-07622.2000. PMID: 11027222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AINS, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. PMID: 18614687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick TJMJ, Altieri SC, Mitchell NE, Wood TL. Synergistic induction of cyclin D1 in oligodendrocyte progenitor cells by IGF-I and FGF-2 requires differential stimulation of multiple signaling pathways. Glia. 2007;55:1011–1022. doi: 10.1002/glia.20520. PMID: 17508424. [DOI] [PubMed] [Google Scholar]
- Furnari FBFT, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. PMID: 17974913. [DOI] [PubMed] [Google Scholar]
- Gan Y1SC, Inge L, Hibner M, Balducci J, Huang Y. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene. 2010;29:4947–4958. doi: 10.1038/onc.2010.240. PMID: 20562913. [DOI] [PubMed] [Google Scholar]
- Goldstein AL, Hannappel E, Kleinman HK. Thymosin beta4: actin-sequestering protein moonlights to repair injured tissues. Trends in molecular medicine. 2005;11:421–429. doi: 10.1016/j.molmed.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Goldstein ALSF, White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin) Proc Natl Acad Sci U S A. 1966;56:1010–1017. doi: 10.1073/pnas.56.3.1010. PMID: 5230175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PABA, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. PMID:18376396. [DOI] [PubMed] [Google Scholar]
- Halterman MWFH. HIF-1alpha and p53 promote hypoxia-induced delayed neuronal death in models of CNS ischemia. Exp Neurol. 1999;159:65–72. doi: 10.1006/exnr.1999.7160. PMID: 10486175. [DOI] [PubMed] [Google Scholar]
- Hayakawa-Yano Y1NK, Fukami S, Gotoh Y, Hirano T, Nakagawa T, Shimazaki T, Okano H. Epidermal growth factor signaling mediated by grb2 associated binder1 is required for the spatiotemporally regulated proliferation of olig2-expressing progenitors in the embryonic spinal cord. Stem Cells. 2007;25:1410–1422. doi: 10.1634/stemcells.2006-0584. PMID: 17332510. [DOI] [PubMed] [Google Scholar]
- Hong LZZX, Zhang HL. p53-mediated neuronal cell death in ischemic brain injury. Neurosci Bull. 2010;26:232–240. doi: 10.1007/s12264-010-1111-0. PMID: 20502500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff T, Muller CS, Otto AM, Netzker R, Hannappel E. beta-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–220. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- Hyun SLJ, Jin H, Nam J, Namkoong B, Lee G, Chung J, Kim VN. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell. 2009;139:1096–1108. doi: 10.1016/j.cell.2009.11.020. PMID: 20005803. [DOI] [PubMed] [Google Scholar]
- Izadi HMA, Bates TC, Olivera ER, Villar-Suarez V, Joshi I, Garg R, Osborne BA, Davis RJ, Rincón M, Anguita J. c-Jun N-terminal kinase 1 is required for Toll-like receptor 1 gene expression in macrophages. Infect Immun. 2007;75:5027–5034. doi: 10.1128/IAI.00492-07. PMID: 17664270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q1ZZ, Ding GL, Silver B, Zhang L, Meng H, Lu M, Pourabdillah-Nejed-D S, Wang L, Savant-Bhonsale S, Li L, Bagher-Ebadian H, Hu J, Arbab AS, Vanguri P, Ewing JR, Ledbetter KA, Chopp M. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage. 2006;32:1080–1089. doi: 10.1016/j.neuroimage.2006.05.025. PMID: 16860575. [DOI] [PubMed] [Google Scholar]
- Jin KMM, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focalcerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001 Apr 10;98(8):4710–4715. doi: 10.1073/pnas.081011098. 98 PMID: 11296300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin KWX, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. PMID: 16924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee STCK, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK, Kim M, Lee SK, Roh JK. MicroRNAs induced during ischemic preconditioning. Stroke. 2010;41:1646–1651. doi: 10.1161/STROKEAHA.110.579649. PMID: 20576953. [DOI] [PubMed] [Google Scholar]
- Li HTJ, Lei H, Cai P, Zhu H, Li B, Xu X, Xia Y, Tang W. Decreased MiR-200a/141 suppress cell migration and proliferation by targeting PTEN in Hirschsprung’s disease. Cell Physiol Biochem. 2014;34:543–553. doi: 10.1159/000363021. PMID: 25116353. [DOI] [PubMed] [Google Scholar]
- Liu XSCM, Wang XL, Zhang L, Hozeska-Solgot A, Tang T, Kassis H, Zhang RL, Chen C, Xu J, Zhang ZG. MicroRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J Biol Chem. 2013a;288:12478–12488. doi: 10.1074/jbc.M112.449025. PMID: 23511639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XSCM, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, Zhang ZG. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One. 2011;6:e23461. doi: 10.1371/journal.pone.0023461. PMID: 21887253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XSCM, Zhang RL, Zhang ZG. MicroRNAs in cerebral ischemia-induced neurogenesis. J Neuropathol Exp Neurol. 2013b;72:718–722. doi: 10.1097/NEN.0b013e31829e4963. PMID 23860031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YLQ, Jia W, Chen J, Wang J, Ye D, Guo X, Chen W, Li G, Wang G, Deng A, Kang J. MicroRNA-200a regulates Grb2 and suppresses differentiation of mouse embryonic stem cells into endoderm and mesoderm. PLoS One. 2013;8:e68990. doi: 10.1371/journal.pone.0068990. PMID: 23874841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Macas JNC, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26:13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. PMID: 17167100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellinghoff IKWM, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, Riggs BL, Horvath S, Liau LM, Cavenee WK, Rao PN, Beroukhim R, Peck TC, Lee JC, Sellers WR, Stokoe D, Prados M, Cloughesy TF, Sawyers CL, Mischel PS. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. PMID: 16282176. [DOI] [PubMed] [Google Scholar]
- Menn BG-VJ, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. PMID:16870736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minger SLEA, Carta EM, Chinoy A, Perry RH, Ballard CG. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007;2:69–74. doi: 10.2217/17460751.2.1.69. PMID: 17465777. [DOI] [PubMed] [Google Scholar]
- Morris DCCM, Zhang L, Lu M, Zhang ZG. Thymosin beta4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience. 2010a;169(2):674–682. doi: 10.1016/j.neuroscience.2010.05.017. 169 PMID: 20627173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DCCM, Zhang L, Zhang ZG. Thymosin beta4: a candidate for treatment of stroke? Ann N Y Acad Sci. 2010b;1194:112–117. doi: 10.1111/j.1749-6632.2010.05469.x. PMID: 20536457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DCCY, Cheung WL, Lu M, Zhang L, Zhang ZG, Chopp M. A dose-response study of thymosin β4 for the treatment of acute stroke. J Neurol Sci. 2014;345:61–67. doi: 10.1016/j.jns.2014.07.006. PMID: 25060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RS1WH, Kinoshita Y, Robbins CA, Donehower LA, Schwartzkroin PA. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci. 1996;16:1337–1345. doi: 10.1523/JNEUROSCI.16-04-01337.1996. PMID: 8778285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatello DKSM, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest. 1998;101:406–412. doi: 10.1172/JCI846. PMID: 9435313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan SPFA, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci. 2009;29:6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. PMID: 19474313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YBXL, Lu Y, Sun X, Yue S, Xiong XX, Giffard RG. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia. 2013;61:1784–1794. doi: 10.1002/glia.22556. PMID: 24038396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YBXL, Yue S, Liu S, Giffard RG. Neuroprotection by astrocytes in brain ischemia: importance of microRNAs. Neurosci Lett. 2014;17:53–58. doi: 10.1016/j.neulet.2013.11.015. PMID: 24269978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey ASP, Jauhari A, Singh T, Khan F, Pant AB, Parmar D, Yadav S. Critical role of the miR-200 family in regulating differentiation and proliferation of neurons. J Neurochem. 2015;133:640–652. doi: 10.1111/jnc.13089. PMID: 25753155. [DOI] [PubMed] [Google Scholar]
- Pang YZB, Fan LW, Rhodes PG, Cai Z. IGF-1 protects oligodendrocyte progenitors against TNFalpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia. 2007;55:1099–1107. doi: 10.1002/glia.20530. PMID: 17577243. [DOI] [PubMed] [Google Scholar]
- Parent JMVZ, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. PMID: 12447935. [DOI] [PubMed] [Google Scholar]
- Rink CKS. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. 2011;43:521–528. doi: 10.1152/physiolgenomics.00158.2010. PMID 20841499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MC, Goldstein AL, Wang YL. Thymosin beta 4 (Fx peptide) is a potent regulator of actin polymerization in living cells. Proc Natl Acad Sci U S A. 1992;89:4678–4682. doi: 10.1073/pnas.89.10.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansal ISW. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. PMID: 15254063. [DOI] [PubMed] [Google Scholar]
- Santra MCM, Zhang ZG, Lu M, Santra S, Nalani A, Santra S, Morris DC. Thymosin β 4 mediates oligodendrocyte differentiation by upregulating p38 MAPK. Glia. 2012;60:1826–1838. doi: 10.1002/glia.22400. PMID: 23073962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra MKM, Zhang RL, Zhang ZG, Meng H, Jiang F, Chopp M. Protection of adult mouse progenitor cells and human glioma cells by de novo decorin expression in an oxygen- and glucose-deprived cell culture model system. J Cereb Blood Flow Metab. 2006a;26:1311–1322. doi: 10.1038/sj.jcbfm.9600285. PMID: 16467781. [DOI] [PubMed] [Google Scholar]
- Santra MLX, Santra S, Zhang J, Zhang RL, Zhang ZG, Chopp M. Ectopic expression of doublecortin protects adult rat progenitor cells and human glioma cells from severe oxygen and glucose deprivation. Neuroscience. 2006b;142:739–752. doi: 10.1016/j.neuroscience.2006.06.065. PMID: 16962712. [DOI] [PubMed] [Google Scholar]
- Santra M, Santra S, Buller B, Santra K, Nallani A, Chopp M. Effect of doublecortin on self-renewal and differentiation in brain tumor stem cells. Cancer Sci. 2011;102:1350–1357. doi: 10.1111/j.1349-7006.2011.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra MSS, Zhang J, Chopp M. Ectopic decorin expression up-regulates VEGF expression in mouse cerebral endothelial cells via activation of the transcription factors Sp1, HIF1alpha, and Stat3. J Neurochem. 2008;105:324–337. doi: 10.1111/j.1471-4159.2007.05134.x. PMID: 1802129. [DOI] [PubMed] [Google Scholar]
- Santra MZZ, Yang J, Santra S, Santra S, Chopp M, Morris DC. Thymosin β4 Upregulation of MicroRNA-146a Promotes Oligodendrocyte Differentiation and Suppression of the Toll-like Proinflammatory Pathway. J Biol Chem. 2014;289:19508–19518. doi: 10.1074/jbc.M113.529966. PMID: 24828499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafidi JHT, Scafidi S, Ritter J, Jablonska B, Roncal M, Szigeti-Buck K, Coman D, Huang Y, McCarter RJ, Jr, Hyder F, Horvath TL, Gallo V. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature. 2014;506:230–234. doi: 10.1038/nature12880. PMID:24390343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack RS1BD, Rosenberg M, Atwal J, Lochmüller H, Aloyz R, Haghighi A, Lach B, Seth P, Cooper E, Miller FD. Adenovirus-mediated gene transfer of the tumor suppressor, p53, induces apoptosis in postmitotic neurons. J Cell Biol. 1996;135:1085–1096. doi: 10.1083/jcb.135.4.1085. PMID: 8922388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanath PRRO, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–518. doi: 10.4161/cc.5.5.2538. PMID: 16552185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trümbach DPN. The conserved miR-8/miR-200 microRNA family and their role in invertebrate and vertebrate neurogenesis. Cell Tissue Res. 2015;359:161–177. doi: 10.1007/s00441-014-1911-z. PMID: 24875007. [DOI] [PubMed] [Google Scholar]
- Wang CJB, Cheng B, Chen J, Bai B. Neuroprotection of microRNA in neurological disorders (Review) Biomed Rep. 2014;2:5. doi: 10.3892/br.2014.297. PMID: 25053999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin HLY, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. PMID: 23630198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong YMA, Meng Y, Zhang Y, Zhang ZG, Morris DC, Chopp M. Treatment of traumatic brain injury with thymosin β in rats. J Neurosurg. 2010;114:102–115. doi: 10.3171/2010.4.JNS10118. PMID: 20486893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong YMA, Meng Y, Zhang Y, Zhang ZG, Morris DC, Chopp M. Neuroprotective and neurorestorative effects of thymosin β4 treatment following experimentaltraumatic brain injury. Ann N Y Acad Sci. 2012a;270:51–58. doi: 10.1111/j.1749-6632.2012.06683.x. PMID: 23050817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong YZY, Mahmood A, Meng Y, Zhang ZG, Morris DC, Chopp M. Neuroprotective and neurorestorative effects of thymosin β4 treatment initiated 6 hours aftertraumatic brain injury in rats. J Neurosurg. 2012b;116:1081–1092. doi: 10.3171/2012.1.JNS111729. PMID: 22324420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DMA, Kyriakis JM. Gene 33 is an endogenous inhibitor of epidermal growth factor (EGF) receptor signaling and mediates dexamethasone-induced suppression of EGF function. J Biol Chem. 2005;280:2924–2933. doi: 10.1074/jbc.M408907200. PMID: 15556944. [DOI] [PubMed] [Google Scholar]
- Xu DPR, Force T, Kyriakis JM. Gene 33/RALT is induced by hypoxia in cardiomyocytes, where it promotes cell death by suppressing phosphatidylinositol 3-kinase and extracellular signal-regulated kinase survival signaling. Mol Cell Biol. 2006;26:5043–5054. doi: 10.1128/MCB.02387-05. PMID: 16782890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W1ZY, Li Y, Wu Z, Zhu D. Myostatin induces cyclin D1 degradation to cause cell cycle arrest through a phosphatidylinositol 3-kinase/AKT/GSK-3 beta pathway and is antagonized by insulin-like growth factor 1. J Biol Chem. 2007 Feb 9;282(6):3799–3808. doi: 10.1074/jbc.M610185200. Epub 2006 Nov 27 282 PMID: 17130121. [DOI] [PubMed] [Google Scholar]
- Yang YAY, Chen Y, Tan X, Guo L, Gibbons DL, Ungewiss C, Peng DH, Liu X, Lin SH, Thilaganathan N, Wistuba II, Rodriguez-Canales J, McLendon G, Creighton CJ, Kurie JM. ZEB1 sensitizes lung adenocarcinoma to metastasis suppression by PI3K antagonism. J Clin Invest. 2014;124:2696–2708. doi: 10.1172/JCI72171. PMID: 24762440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CCM, Cui Y, Wang L, Zhang R, Zhang L, Lu M, Szalad A, Doppler E, Hitzl M, Zhang ZG. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J Neurosci Res. 2010;88:3275–3281. doi: 10.1002/jnr.22495. PMID: 20857512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZZ, Morris D, Li Y, Roberts C, Elias SB, Chopp M. Neurological functional recovery after thymosin beta4 treatment in mice with experimental auto encephalomyelitis. Neuroscience. 2009;164:1887–1893. doi: 10.1016/j.neuroscience.2009.09.054. PMID: 19782721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LCM, Meier DH, Winter S, Wang L, Szalad A, Lu M, Wei M, Cui Y, Zhang ZG. Sonic hedgehog signaling pathway mediates cerebrolysin-improved neurological function after stroke. Stroke. 2013;44:1965–1972. doi: 10.1161/STROKEAHA.111.000831. PMID: 23696546. [DOI] [PubMed] [Google Scholar]
- Zhang LCM, Zhang RL, Wang L, Zhang J, Wang Y, Toh Y, Santra M, Lu M, Zhang ZG. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PLoS One. 2010;5:e11016. doi: 10.1371/journal.pone.0011016. PMID: 20552017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RCM, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013;7:201. doi: 10.3389/fncel.2013.00201. PMID: 24194700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RZZ, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004a;24:441–448. doi: 10.1097/00004647-200404000-00009. PMID: 15087713. [DOI] [PubMed] [Google Scholar]
- Zhang RZZ, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci. 2004b;24:5810–5815. doi: 10.1523/JNEUROSCI.1109-04.2004. PMID: 15215303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Roberts C, Wei M, Wang X, Liu X, Lu M, Zhang ZG. Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoSONE. 2012;7:e48141. doi: 10.1371/journal.pone.0048141. PMID: 23118941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Roberts C, Jia L, Wei M, Lu M, Wang X, Pourabdollah S, Zhang ZG. Ascl1 lineage cells contribute to ischemia-induced neurogenesis and oligoden- drogenesis. J. Cereb. Blood Flow Metab. 2011;31:614–625. doi: 10.1038/jcbfm.2010.134. PMID: 20736965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RLZZ, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001a;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. PMID: 11483298. [DOI] [PubMed] [Google Scholar]
- Zhang RLZZ, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001b;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. PMID:11483298. [DOI] [PubMed] [Google Scholar]
- Zhang XPK, Bose R, Jura N, Cole PA, Kuriyan J. Inhibition of the EGF receptor by binding of MIG6 to an activating kinase domain interface. Nature. 2007;450:741–744. doi: 10.1038/nature05998. PMID:18046415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YUY, Liu XS, Buller B, Wang X, Chopp M, Zhang ZG. The MicroRNA-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J Neurosci. 2013;33:6885–6894. doi: 10.1523/JNEUROSCI.5180-12.2013. PMID: 23595747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZGCM. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. PMID: 19375666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BPLY, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–982. doi: 10.1038/ncb1101-973. PMID: 11715018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.