Abstract
Objective
Phentermine is thought to cause weight loss through a reduction in hunger. We hypothesized that higher hunger ratings would predict greater weight loss with phentermine.
Design and Methods
This is an observational pilot study in which all subjects were treated with phentermine for 8 weeks and appetite and eating behaviors were measured at baseline and week 8. Outcomes were compared in subjects with ≥5% vs <5% weight loss, and linear regression was used to identify predictors of percent weight loss.
Results
27 subjects (37 ± 4.5 yrs, 93.8 ± 12.1 kg, BMI 33.8 ± 3.1 kg/m2) completed the study, with mean weight loss of -5.4 ± 3.3 kg (-5.7 ± 3.2%). Subjects with ≥5% weight loss had higher baseline pre-breakfast hunger (p=0.017), desire to eat (p=0.003), and prospective food consumption (0.006), and lower baseline cognitive restraint (p=0.01). In addition, higher baseline home prospective food consumption (p=0.002) and lower baseline cognitive restraint (p<0.001) were found to be predictors of weight loss.
Conclusion
These results suggest that individuals reporting greater hunger and less restraint are more likely to achieve significant weight loss with phentermine. This information can be used clinically to determine who might benefit most from phentermine treatment.
Keywords: phentermine, appetite, eating behavior, pharmacotherapy
Introduction
The increasing prevalence of obesity worldwide has focused attention on weight loss methods (1). Weight loss achieved by changes in diet and physical activity are the cornerstones in the treatment of obesity (2), but weight control methods often produce only short-term success (3, 4, 5).
Pharmacological therapy has been proposed as an adjunct to lifestyle changes to improve weight loss in people with obesity and overweight individuals with metabolic complications (2). Phentermine is the most prescribed weight loss drug approved for short-term use (less than 12 weeks) by the Food and Drug Administration (FDA), and has been used steadily since its initial approval in 1959 (6, 7). Phentermine is thought to reduce hunger by stimulating the release of norepinephrine in the hypothalamus (8), and is indicated as an adjunct to lifestyle modification in individuals with a body mass index (BMI) of ≥ 30 kg/m2, or 27 kg/m2 in the presence of co-morbidities (hypertension, diabetes, hyperlipidemia) (7).
Studies on the effect of phentermine alone on weight reduction have shown a mean weight loss of approximately 5% over a short-term period (no studies of safety or efficacy beyond one year have been done) (9, 10, 11, 12). While increased heart rate and blood pressure are often cited as potential adverse effects (13, 14), a number of studies have shown decreases in blood pressure (12, 15, 16), likely related to weight loss (although the decrease is generally less than that achieved by patients who achieve similar weight loss on placebo) (17). A South Korean group performed post-marketing surveillance research on phentermine, finding that while adverse events due to phentermine were very common (30%), in most cases they were mild (insomnia and dry mouth), (18). However, the two most recent randomized controlled trials showed a 23-47% drop-out rate, due to adverse events, lack of efficacy, or other unspecified reasons. These studies both found that around 85% of phentermine-treated patients achieved a 5% weight loss, and approximately 50% achieved a 10% weight loss (16, 19).
Since not all treated patients are able to achieve a clinically meaningful degree of weight loss, and many patients do not tolerate the medication, it is important clinically to find predictors of response to treatment with phentermine. As phentermine is thought to cause weight loss through suppression of hunger, it could be hypothesized that those individuals who eat excessively due to hunger (as opposed to emotional stress, boredom, etc.) might have a better weight loss response. However, only one study has made any mention of decreased hunger in phentermine-treated patients (15), and this was anecdotal.
To address this question, we designed an observational pilot study in which all participants were treated with phentermine for 8 weeks, and subjective ratings of appetite were measured at baseline and at the end of the study. Our primary hypothesis was that subjective measures of hunger (hunger, desire to eat, and prospective food consumption) would decrease after 8 weeks of treatment with phentermine. In addition, we hypothesized that greater hunger at baseline would predict weight loss with phentermine.
Methods and procedures
Ethics Statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Colorado Multiple Institutional Review Board. All patients provided written informed consent for the collection of samples and subsequent analysis.
Subjects
Healthy men and women between the ages of 30-45 years of age, with BMI between 30-40 kg/m2 were recruited through e-mail flyers distributed to the medical campus. Exclusion criteria were previous bariatric surgery for weight loss, current treatment with medications affecting appetite (weight loss or psychiatric medications), history of eating disorder, substance abuse, diabetes, cardiovascular disease, serum creatinine >1.5 mg/dL, AST or ALT >2 times upper limit of normal, inadequately controlled hypertension, hypothyroidism, or hyperlipidemia (LDL>160 mg/dL or TG>400 mg/dL). Female subjects were required to use adequate contraception for the duration of the study.
Methods
All participants were initially screened by phone and then underwent a screening visit which took place at the Clinical and Translational Research Center (CTRC). During this visit, the consent was signed, a history and physical was performed, and height, weight, and vital signs were measured. Weight was measured and recorded to the nearest 0.1 kg using the same scale, with participants lightly clothed and without shoes, one time at the screening visit and at each follow-up visit. Height was measured to the nearest 0.1 cm using a stadiometer once at the screening visit only. Waist circumference was measured once just above the iliac crest and recorded to the nearest 0.1 cm (20). Before having their blood pressure and pulse taken, participants were asked to sit for 5 minutes. They then had blood drawn (fasting plasma glucose, electrolytes, blood urea nitrogen and creatinine, HgbA1c, lipids, TSH, AST and ALT) and completed the Eating Attitudes Test (EAT-26) to screen for eating disorder risk (individuals with a score >20 were excluded) (21). They were also provided with appetite ratings to be performed at home using visual analog scales (VAS). Hunger was rated on a 100-mm line preceded by the question, “How hungry do you feel?” and anchored by “not at all hungry” on the left and “extremely hungry” on the right. Satiety was rated by VAS with the question, “How full do you feel?” with the anchors “not at all” and “extremely.” Desire to eat was rated by VAS with the question, “How strong is your desire to eat?” with the anchors “very weak” and “very strong.” Prospective food consumption (PFC) was rated by VAS with the question “How much food do you think you could eat right now?” with the anchors “nothing at all” and “large amount” (22). Participants were asked to rate their appetite at home prior to breakfast, lunch and dinner for 3 days, and to bring these ratings to their baseline study visit.
After confirming eligibility and continued interest in the study, all participants came to the CTRC for a baseline visit. They were asked to arrive in the morning after fasting for at least 10 hours and without vigorous physical activity during the preceding 24 hours. All women were given a pregnancy test to confirm they were not pregnant, and all participants had weight, waist circumference, and vital signs measured. They then completed the 51-item Three-Factor Eating Questionnaire (TFEQ). The TFEQ is a questionnaire that assesses three principal human eating behaviors, with higher scores in each domain denoting higher dietary restraint, disinhibited eating, and predisposition to hunger (23). Participants were asked to rate their appetite as described above for home appetite ratings (hunger, satiety, desire to eat, and PFC) prior to breakfast. They were then given a breakfast meal which provided 30% of estimated caloric needs (50% carbohydrate, 15% protein and 35% fat). Caloric needs were estimated with the use of the Mifflin-St Jeor Equations: Males: RMR = (9.99 × weight kg) + (6.25 × height cm) − (4.92 × age) + 5; Females: RMR = (9.99 × weight kg) + (6.25 × height cm) − (4.92 × age) − 161. The content of the breakfast meal was tailored to each participant's food preferences, as identified with the use of a standard food preferences survey administered by the CTRC Nutrition Core. Most individuals were given eggs, toast, and fruit. They were then asked to rate their appetite as described above every 10 minutes for one hour following consumption of the meal. They also were asked to rate the palatability of the meal using the question “how palatable was your meal?” and anchored by “not at all” on the left and “very” on the right (24). Palatability was rated for the purpose of excluding participants who found the meal unpalatable (<50 mm on VAS), but none were excluded for this reason. After completing these procedures, participants were given an 8-week supply of phentermine hydrochloride 30 mg tablets (ANDA 091359). They were instructed to take one pill at the same time each morning. They were instructed on potential side effects and were given contact information for study personnel to report any adverse effects. They were then asked to return at 1, 3, and 5 weeks after starting medication for follow up visits. At these visits, compliance and side effects were assessed by verbal questioning, and weight, waist circumference, blood pressure and heart rate were measured. Reasons for discontinuing the medication included: Rise in arterial blood pressure values >15 mmHg in systolic and/or 8 mm Hg in diastolic pressure, or blood pressure values that were >140/90 on therapy; rise in resting heart rate >15 beats per minute; clinically important deterioration of the subject's medical status; severe insomnia; frequent or bothersome palpitations; the investigator believed it was in the best interest of the participant; or the participant requested withdrawal from the study. Participants were asked to repeat home appetite ratings as described above for 3 days between 5 and 8 weeks, and returned at week 8 for a final visit. They were asked to bring pill bottles to confirm compliance with the study medication, as well as home appetite ratings. The 8 week visit replicated the first visit without a blood draw. They repeated the TFEQ and breakfast and appetite ratings as described previously. The meal consisted of the exact foods they were given at their baseline visit but caloric needs were recalculated using their current body weight, such that the meal was smaller if weight loss was achieved. The study was completed when the breakfast meal and appetite ratings were finished.
Statistical analysis
Sample size was determined based on a study that examined the reproducibility, power and validity of VAS in assessment of appetite sensations in single test meal studies (24). For an unpaired design, 27 subjects were required to achieve 80% power to detect a 10 mm difference in PFC.
All analyses are for participants completing the full 8-week study. Analysis of the change in participant characteristics (weight, heart rate, blood pressure and waist circumference) and pre-breakfast hunger ratings (hunger, desire and PFC) from baseline to week 8 was performed using paired t-tests. For satiety, the area under the curve (AUC) from time zero to 60 minutes was calculated using the trapezoid method (25), and AUC at baseline and 8 weeks was compared with the use of a paired t-test. For all home appetite ratings, the mean value for each rating over the 3-day period at baseline was compared to the mean value over the 3-day period at 8 weeks with the use of paired t-tests. All statistical tests were two-tailed with significance set at p<0.05.
Participants were then split into groups based on percent weight loss (≥5% and <5% weight loss). Baseline characteristics of these two groups as well as baseline measures of appetite were compared using two-tailed t-tests with significance set at p<0.05.
Linear regression was used to model the effects of baseline predictors and change from baseline to week 8 predictors on percent weight loss. Potential predictors were tested for pairwise correlation. Due to high correlation among baseline measures of hunger (hunger, desire and PFC) and change from baseline to week 8 for these measures of hunger, only one predictor (PFC) was chosen for use in linear regression. Single predictor models were fit for all potential predictors, which included baseline BMI and baseline values and changes from baseline values for home PFC, pre-breakfast PFC, and TFEQ variables. Any predictor that was significant at the 0.10 alpha level was considered a candidate for the final model. A multiple-predictor model was then fit with the candidate baseline predictors listed above. Additionally, a multiple-predictor model was fit with all candidate change from baseline predictors. Backwards elimination was used to remove non-significant predictors from the final models one at a time, starting with the least significant predictor, until only significant predictors remained in the final model. A composite model, containing all baseline and change from baseline predictors, gave the same results as the change from baseline model after backwards elimination. Thus, the results of this model are not included here.
Results
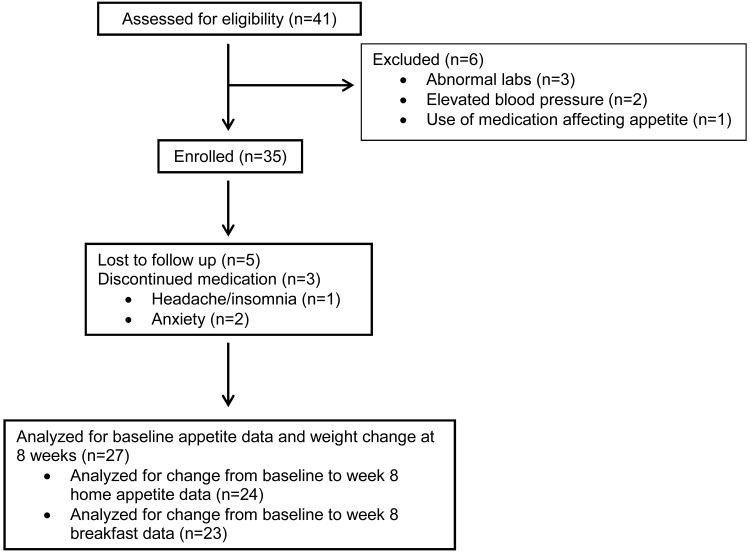
A total of 41 individuals were screened, 35 were enrolled, and 27 completed the study (see Figure). The mean age was 37 ± 4.5 years, mean weight 93.8 ± 12.1 kg, and mean BMI was 33.8 ± 3.1 kg/m2 (see Table 1). The majority of subjects (n=24) were female.
Figure.
Flow of participants through screening, enrollment, and follow-up.
Table 1. Participant Characteristics1.
| Age (years) | 37 ± 4.5 | |
| Gender (n) | Female | 24 |
| Male | 3 | |
| Race (n) | White | 22 |
| African American | 4 | |
| Other | 1 | |
| Ethnicity (n) | Non-Hispanic | 16 |
| Hispanic | 9 | |
| Other | 2 | |
| Baseline weight (kg) | 93.8 ± 12.1 | |
| Baseline BMI (kg/m2) | 33.8 ± 3.1 |
Values are means ± standard deviation, n=27
First, the change from baseline to week 8 was examined for all outcomes for participants completing the study (n=27) (see Table 2). The mean weight loss was -5.4 ± 3.3 kg, or -5.7 ± 3.2%. Weight, BMI, and waist circumference all decreased significantly. Blood pressure did not change from baseline at weeks 1, 3, 5 or 8, but heart rate increased at week 8 by 8.6 (4.7, 12.5) beats per minute (p<0.001), and to a similar degree at weeks 1, 3 and 5 (data not shown). While pre-breakfast hunger and post-breakfast satiety were unchanged, pre-breakfast desire and PFC both decreased slightly with phentermine (p=0.05 for both). Hunger as measured with the TFEQ decreased (p<0.001). Cognitive restraint (CR) increased (p<0.001) and disinhibition decreased (p<0.001) with phentermine as well. In addition, mean pre-meal hunger (measured at home) decreased (p<0.001), as did mean pre-meal desire (p<0.001) and mean pre-meal PFC (p=0.001). Pre-meal satiety measured at home was unchanged with phentermine treatment.
Table 2. Baseline and 8 week anthropometric and appetite data1,2.
| Baseline | 8 week | Change | p-value | |
|---|---|---|---|---|
| Weight (kg) | 93.8 ± 12.1 | 88.4 ± 11.4 | -5.4 (-6.7, -4.1) | <0.001 |
| BMI (kg/m2) | 33.8 ± 3.1 | 31.8 ± 2.8 | -2.0 (-2.4, -1.5) | <0.001 |
| Waist circumference (cm) | 101.3 ± 9.4 | 97.2 ± 7.8 | -4.0 (-6.3, -1.7) | 0.002 |
| Heart rate (BPM) | 64.8 ± 11.0 | 73.4 ± 12.3 | 8.6 (4.7, 12.5) | <0.001 |
| SBP (mm/Hg) | 121.7 ± 8.7 | 122.5 ± 8.8 | 0.9 (-2.3, 4.1) | 0.59 |
| DBP (mm/Hg) | 78.0 ± 8.5 | 77.6 ± 9.7 | -0.4 (-4.0, 3.3) | 0.83 |
| Pre-breakfast hunger (mm)3 | 67.5 ± 24.2 | 55.0 ± 27.5 | -12.5 (-29.5, 4.4) | 0.13 |
| Post-breakfast satiety AUC3 | 3909 ± 1114 | 4013 ± 1497 | 103 (-568, 775) | 0.75 |
| Pre-breakfast desire (mm)3 | 70.0 ± 25.0 | 54.0 ± 29.2 | -16.0 (-32.1, 0.06) | 0.05 |
| Pre breakfast PFC (mm)3 | 69.0 ± 23.6 | 55.0 ± 22.6 | -14.0 (-28.3, 0.2) | 0.05 |
| TFEQ – Hunger | 6.7 ± 3.3 | 3.0 ± 2.9 | -3.7 (-5.4, -2.0) | <0.001 |
| TFEQ – Cognitive Restraint | 9.8 ± 5.2 | 13.1 ± 4.4 | 3.3 (1.5, 5.0) | <0.001 |
| TFEQ – Disinhibition | 8.8 ± 3.5 | 5.7 ± 2.9 | -3.1 (-4.3, -2.0) | <0.001 |
| Mean pre-meal hunger (mm)4 | 62.2 ± 12.4 | 45.9 ± 18.1 | -16.2 (-25.0, -7.6) | <0.001 |
| Mean pre-meal satiety (mm)4 | 35.7 ± 10.7 | 35.6 ± 15.2 | -0.1 (-6.0, 5.8) | 0.97 |
| Mean pre-meal desire (mm)4 | 62.2 ± 16.5 | 37.0 ± 20.2 | -25.1 (-34.9, -15.3) | <0.001 |
| Mean pre-meal PFC (mm)4 | 59.5 ± 16.9 | 41.7 ± 18.7 | -17.8 (-27.8, -7.8) | 0.001 |
BPM, beats per minute; SBP, systolic blood pressure; DBP, diastolic blood pressure; AUC, area under the curve; PFC, prospective food consumption; TFEQ, three factor eating questionnaire
Values for baseline and 8 week data are means ± standard deviation, values for change are mean difference (95% confidence interval), n=27
n=23
n=24
Next, baseline measures of appetite and eating behaviors were examined in the group of participants who achieved weight loss ≥5% versus those who achieved <5% weight loss (see Table 3). These groups were not different with respect to baseline characteristics (weight, BMI, heart rate, blood pressure, gender, race or ethnicity). However, baseline pre-breakfast hunger (p=0.017), desire to eat (p=0.003) and PFC (p=0.006) were greater in the group that achieved ≥5% weight loss vs. the group that achieved <5% weight loss. Baseline pre-breakfast satiety and home appetite ratings were not found to differ between groups. Disinhibition and hunger (as measured with TFEQ) did not differ between groups, but baseline CR was lower (p=0.01) in the group achieving ≥5% weight loss.
Table 3. Baseline home and pre-breakfast appetite ratings in subjects with weight loss less than 5% as compared to subjects with weight loss greater than or equal to 5%1,2.
| Weight loss <5% (n=11) | Weight loss ≥5% (n=16) | p-value | |
|---|---|---|---|
| Home hunger (mm) | 62.2 (53.9, 70.5) | 61.1 (54.5, 67.7) | 0.828 |
| Home satiety (mm) | 34.2 (25.2, 43.2) | 40.4 (33.2, 47.5) | 0.279 |
| Home desire (mm) | 63.8 (52.9, 74.8) | 62.4 (53.7, 71.1) | 0.833 |
| Home PFC (mm) | 58.0 (46.4, 69.6) | 62.9 (53.7, 72.0) | 0.501 |
| Pre-breakfast hunger (mm) | 56.7 (43.7, 69.8) | 77.8 (67.0, 88.6) | 0.017 |
| Satiety AUC (mm × 60 min) | 3690 (2950, 4430) | 3910 (3290, 4530) | 0.647 |
| Pre-breakfast desire (mm) | 54.7 (42.1, 67.3) | 80.4 (69.9, 90.8) | 0.004 |
| Pre-breakfast PFC (mm) | 55.9 (43.7, 68.2) | 78.9 (68.8, 89.1) | 0.006 |
| TFEQ cognitive restraint | 12.8 (9.92, 15.7) | 7.75 (5.35, 10.2) | 0.010 |
| TFEQ disinhibition | 8.64 (6.38, 10.9) | 8.94 (7.07, 10.8) | 0.834 |
| TFEQ hunger | 6.09 (4.00, 8.18) | 7.19 (5.45, 8.92) | 0.414 |
PFC, prospective food consumption; AUC, area under curve; TFEQ, three factor eating questionnaire
Values are means (95% confidence interval)
Next, linear regression was used to model the effects of baseline predictors and change from baseline to week 8 predictors on percent weight loss. Potential predictors were tested for pairwise correlation. There was high correlation among the three baseline pre-breakfast measures of hunger (hunger, desire to eat and PFC), among the change from baseline pre-breakfast hunger measures, between baseline home PFC and baseline home desire, and between change from baseline home PFC and change from baseline home desire. Thus, only one predictor (PFC) for pre-breakfast appetite ratings and home appetite ratings was included in the model. Of the baseline predictors, baseline home PFC, baseline CR, and baseline pre-breakfast PFC had p-values less than 0.10 and were considered candidates for the final baseline model. Additionally, baseline BMI was considered a candidate for the final model for scientific reasons, as BMI may impact appetite. After non-significant predictors were removed one at a time, the final model contained only three significant predictors: baseline BMI (p=0.042), baseline home PFC (p<0.001) and baseline CR (p=0.002) (see Table 4). Specifically, for every 1 unit increase in baseline BMI, weight loss increased by 0.294% (0.011%, 0.576%), and for every 1 mm increase in baseline home PFC, weight loss increased by 0.084% (0.033%, 0.134%). Also, for every 1-unit decrease in baseline CR, weight loss increased by 0.338% (0.169%, 0.508%).
Table 4. Parameter estimates, confidence intervals, and p-values for final model of baseline predictors of weight loss1,2.
| Estimate (95% CI) | p-Value | |
|---|---|---|
| Baseline cognitive restraint | -0.338 (-0.508, -0.169) | <0.001 |
| Baseline home PFC | 0.084 (0.033, 0.134) | 0.002 |
| Baseline BMI | 0.294 (0.011, 0.576) | 0.042 |
PFC, prospective food consumption
Estimate is percent change in weight loss for every 1 unit increase in value of variable; n=26
Of the change predictors, change in CR, disinhibition, and hunger (measured with TFEQ), change in home PFC and change in pre-breakfast PFC had p-values less than 0.10 and were considered candidates for the final change from baseline model. After non-significant predictors were removed one at a time, the final model contained only two significant predictors: change in pre-breakfast PFC (p=0.017) and change in CR (p=0.026) (see Table 5). Specifically, for every 1 mm loss from baseline in pre-breakfast PFC, weight loss increased by 0.046% (0.009%, 0.082%). Also, for every one unit gain from baseline in TFEQ CR, weight loss increased by 0.304% (0.040%, 0.569%).
Table 5. Parameter estimates, confidence intervals, and p-values for final model of change (baseline to week 8) predictors of weight loss1,2.
| Estimate (95%CI) | p-Value | |
|---|---|---|
| Change in pre-breakfast PFC | -0.046 (-0.082, -0.009) 0.304 | 0.017 |
| Change in cognitive restraint | (0.040, 0.569) | 0.026 |
PFC, prospective food consumption
Estimate is percent change in weight loss for every 1 unit increase in value of variable; n=23
Discussion
In this study, treatment with phentermine for 8 weeks was found to decrease several measures of hunger, to increase CR, and to decrease disinhibition. Moreover, individuals who were able to achieve ≥ 5% weight loss had higher baseline pre-breakfast hunger, desire to eat and PFC, and lower CR. In addition, higher baseline PFC and greater reduction after treatment, and lower baseline CR and greater increase after treatment were all found to be predictors of greater weight loss.
Measures of appetite and eating behaviors have been developed for use in obesity research to evaluate potential contributors to weight gain. Subjective ratings of appetite as measured using VAS have been shown in most studies to predict subsequent energy intake and indicate potential changes in body weight, and are frequently used in studies assessing appetite and the appetitive response to various interventions (22, 24, 26, 27, 28, 29). In addition, behavioral traits assessed with tools such as the TFEQ have been studied with respect to BMI and propensity to weight gain. High levels of hunger and disinhibition have been shown to correlate positively with BMI (30, 31, 32, 33), while high cognitive restraint appears to reduce the degree of weight gain over time (33, 34).
While appetite expression and eating behaviors undoubtedly play an important role in the development of obesity, evaluation of the effects of anti-obesity drugs on these factors has been lacking (35). Although anecdotal evidence suggests that phentermine reduces hunger, no studies have assessed the effects of phentermine on subjective measures of appetite. The present study shows that phentermine treatment results in significant reductions in several different measures of hunger, and that higher PFC at baseline is a predictor of greater weight loss with treatment. In addition, those individuals with higher ratings of hunger at baseline were more likely to achieve ≥5% weight loss (a threshold chosen for clinical relevance, as cardiovascular and metabolic risk factors are improved with this degree of weight loss (36)). This information can be used clinically to help determine which patients should be treated with phentermine, as individuals who are unable to lose weight due to hunger when restricting calories are likely to benefit most from treatment. Alternatively, those individuals who do not report high levels of hunger are less likely to benefit from phentermine, so other treatments might be considered in such patients.
Interestingly, phentermine treatment was also found to increase CR and to reduce disinhibition. This suggests effects of treatment outside of the expected effects on hunger. One other study has suggested similar effects of phentermine treatment, through anecdotal reports of patients describing “stronger control of eating, diminution or absence of food cravings, or improved ability to follow their eating plan” (15). These effects on eating behavior are likely reflected in part by our findings of reduced disinhibition and increased CR with phentermine treatment. This information also can be clinically useful, as those individuals with low restraint (defined as the conscious restriction of food intake to prevent weight gain or promote weight loss) may be more likely to benefit from treatment with phentermine, while patients who already feel they are restrained eaters are less likely to benefit from treatment.
There are a number of limitations to this study that should be addressed. First, this study was not a randomized controlled trial, so effects on appetite are presumed, but cannot definitively be ascribed to phentermine treatment, as weight loss alone may cause changes in appetite and eating behaviors. However, the aim of the study was to identify patterns of appetite expression and eating behaviors that could predict greater response to treatment. Next, our study sample includes a greater number of women than men, so it is uncertain whether the results are applicable also to men. However, we did not find any gender differences with respect to baseline or 8 week appetite ratings and scores on the TFEQ. In addition, it could be argued that appetite ratings (pre-breakfast and home measures) might vary within an individual depending on the most recent meal eaten. However, these measures were meant to assess free-living appetite and therefore meal content and timing could not be controlled. Lastly, the decisions to use only pre-meal hunger measures and only one of the three hunger measures (PFC) in the linear regression analysis could be considered to be limitations. However, because these variables were highly correlated, there was a high risk of finding confounding, effect modification, or mediators in the final model, and so only one of these variables could be used. We decided to use pre-meal hunger ratings because they have been shown to correlate with subsequent food intake (26) and we chose PFC because it was highly correlated with both pre-breakfast ratings (hunger and desire to eat) and home ratings (desire to eat), and was felt to be a more concrete assessment of energy intake.
Overall, this study showed that individuals with greater hunger and less restraint were more likely to achieve clinically meaningful weight loss. This information can be used clinically to better predict which patients will achieve greater weight loss with phentermine treatment.
What is already known about this subject
Phentermine is thought to cause weight loss through reductions in hunger and subsequent energy intake
The degree of weight loss with phentermine treatment is widely variable
The ability to predict weight loss success with phentermine for individual patients would be clinically useful
What this study adds
Treatment with phentermine for 8 weeks was found to decrease several measures of hunger, to increase cognitive restraint, and to decrease disinhibition
Individuals who were able to achieve ≥ 5% weight loss with phentermine treatment had higher baseline (pre-treatment) hunger prior to breakfast and lower cognitive restraint
Higher baseline hunger and greater reduction after treatment, and lower baseline cognitive restraint and greater increase after treatment were all found to be predictors of greater weight loss
Acknowledgments
We acknowledge and thank the University of Colorado Clinical and Translational Science Institute (CCTSI) as well as the CCTSI Metabolic Kitchen for their support of this study. We would also like to thank the individuals who participated in this study.
Funding sources: This publication was supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780.
Footnotes
Conflicts of interest: All authors declare no conflict of interest.
Author contributions: RHE, AF, EAT and MAC contributed to the design of the study. JLB and EAT carried out the study procedures and collected data. BM and EAT performed data analysis. All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayyad C, Andersen T. Long-term efficacy of dietary treatment of obesity: a systematic review of studies published between 1931 and 1999. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2000;1:113–119. doi: 10.1046/j.1467-789x.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- 4.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12 Suppl:151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH. Clinical practice. Nonsurgical management of obesity in adults. The New England journal of medicine. 2008;358:1941–1950. doi: 10.1056/NEJMcp0801652. [DOI] [PubMed] [Google Scholar]
- 6.Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Annals of internal medicine. 2005;143:380–385. doi: 10.7326/0003-4819-143-5-200509060-00013. [DOI] [PubMed] [Google Scholar]
- 7.Stafford RS, Radley DC. National trends in antiobesity medication use. Archives of internal medicine. 2003;163:1046–1050. doi: 10.1001/archinte.163.9.1046. [DOI] [PubMed] [Google Scholar]
- 8.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. Br Med J. 1968;1:352–354. doi: 10.1136/bmj.1.5588.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truant AP, Olon LP, Cobb S. Phentermine resin as an adjunct in medical weight reduction: a controlled, randomized, double-blind prospective study. Current therapeutic research, clinical and experimental. 1972;14:726–738. [PubMed] [Google Scholar]
- 11.Campbell CJ, Bhalla IP, Steel JM, Duncan LJ. A controlled trial of phentermine in obese diabetic patients. The Practitioner. 1977;218:851–855. [PubMed] [Google Scholar]
- 12.Valle-Jones JC, Brodie NH, O'Hara H, O'Hara J, McGhie RL. A comparative study of phentermine and diethylpropion in the treatment of obese patients in general practice. Pharmatherapeutica. 1983;3:300–304. [PubMed] [Google Scholar]
- 13.Bray GA. Lifestyle and pharmacological approaches to weight loss: efficacy and safety. The Journal of clinical endocrinology and metabolism. 2008;93:S81–88. doi: 10.1210/jc.2008-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan LM. Pharmacologic therapies for obesity. Gastroenterology clinics of North America. 2010;39:69–79. doi: 10.1016/j.gtc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Hendricks EJ, Greenway FL, Westman EC, Gupta AK. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity (Silver Spring) 2011;19:2351–2360. doi: 10.1038/oby.2011.94. [DOI] [PubMed] [Google Scholar]
- 16.Kim KK, Cho HJ, Kang HC, Youn BB, Lee KR. Effects on weight reduction and safety of short-term phentermine administration in Korean obese people. Yonsei medical journal. 2006;47:614–625. doi: 10.3349/ymj.2006.47.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan DH, Bray GA. Pharmacologic treatment options for obesity: what is old is new again. Current hypertension reports. 2013;15:182–189. doi: 10.1007/s11906-013-0343-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim HO, Lee JA, Suh HW, Kim YS, Kim BS, Ahn ES, et al. Postmarketing surveillance study of the efficacy and safety of phentermine in patients with obesity. Korean journal of family medicine. 2013;34:298–306. doi: 10.4082/kjfm.2013.34.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes, obesity & metabolism. 2010;12:876–882. doi: 10.1111/j.1463-1326.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 21.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychological medicine. 1982;12:871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- 22.Cornier MA, Grunwald GK, Johnson SL, Bessesen DH. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite. 2004;43:253–259. doi: 10.1016/j.appet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of psychosomatic research. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 24.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 25.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes care. 1995;18:245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 26.Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. The British journal of nutrition. 2000;84:405–415. doi: 10.1017/s0007114500001719. [DOI] [PubMed] [Google Scholar]
- 27.Drapeau V, Blundell J, Therrien F, Lawton C, Richard D, Tremblay A. Appetite sensations as a marker of overall intake. The British journal of nutrition. 2005;93:273–280. doi: 10.1079/bjn20041312. [DOI] [PubMed] [Google Scholar]
- 28.Drapeau V, King N, Hetherington M, Doucet E, Blundell J, Tremblay A. Appetite sensations and satiety quotient: predictors of energy intake and weight loss. Appetite. 2007;48:159–166. doi: 10.1016/j.appet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Thomas EA, Bechtell JL, Vestal BE, Johnson SL, Bessesen DH, Tregellas JR, et al. Eating-related behaviors and appetite during energy imbalance in obese-prone and obese-resistant individuals. Appetite. 2013;65C:96–102. doi: 10.1016/j.appet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dykes J, Brunner EJ, Martikainen PT, Wardle J. Socioeconomic gradient in body size and obesity among women: the role of dietary restraint, disinhibition and hunger in the Whitehall II study. Int J Obes Relat Metab Disord. 2004;28:262–268. doi: 10.1038/sj.ijo.0802523. [DOI] [PubMed] [Google Scholar]
- 31.Provencher V, Drapeau V, Tremblay A, Despres JP, Lemieux S. Eating behaviors and indexes of body composition in men and women from the Quebec family study. Obes Res. 2003;11:783–792. doi: 10.1038/oby.2003.109. [DOI] [PubMed] [Google Scholar]
- 32.Aurelie L, Gilles F, Jean-Jacques D, Agathe A, Sophie V, Daniel T, et al. Characterization of the Three-Factor Eating Questionnaire scores of a young French cohort. Appetite. 2012;59:385–390. doi: 10.1016/j.appet.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Hays NP, Bathalon GP, McCrory MA, Roubenoff R, Lipman R, Roberts SB. Eating behavior correlates of adult weight gain and obesity in healthy women aged 55-65 y. Am J Clin Nutr. 2002;75:476–483. doi: 10.1093/ajcn/75.3.476. [DOI] [PubMed] [Google Scholar]
- 34.Williamson DA, Lawson OJ, Brooks ER, Wozniak PJ, Ryan DH, Bray GA, et al. Association of body mass with dietary restraint and disinhibition. Appetite. 1995;25:31–41. doi: 10.1006/appe.1995.0039. [DOI] [PubMed] [Google Scholar]
- 35.Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA. Pharmacological management of appetite expression in obesity. Nature reviews Endocrinology. 2010;6:255–269. doi: 10.1038/nrendo.2010.19. [DOI] [PubMed] [Google Scholar]
- 36.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]