Abstract
Mutations in endoglin, a TGFβ/BMP coreceptor, are causal for hereditary hemorrhagic telangiectasia (HHT). Endoglin-null (Eng−/−) mouse embryos die at embryonic day (E)10.5-11.5 due to defects in angiogenesis. In part, this is due to an absence of vascular smooth muscle cell differentiation and vessel investment. Prior studies from our lab and others have shown the importance of endoglin expression in embryonic development in both endothelial cells and neural crest stem cells. These studies support the hypothesis that endoglin may play cell-autonomous roles in endothelial and vascular smooth muscle cell precursors. However, the requirement for endoglin in vascular cell precursors remains poorly defined. Our objective was to specifically delete endoglin in neural crest- and somite-derived Pax3-positive vascular precursors to understand the impact on somite progenitor cell contribution to embryonic vascular development. Pax3Cre mice were crossed with Eng+/− mice to obtain compound mutant Pax3Cre/+;Eng+/− mice. These mice were then crossed with homozygous endoglin LoxP-mutated (EngLoxP/LoxP) mice to conditionally delete the endoglin gene in specific lineages that contribute to endothelial and smooth muscle constituents of developing embryonic vessels. Pax3Cre/+;EngLoxP/− mice showed a variety of vascular defects at E10.5, and none of these mice survived past E12.5. Embryos analyzed at E10.5 showed malformations suggestive of misdirection of the intersomitic vessels. The dorsal aorta showed significant dilation with associated vascular smooth muscle cells exhibiting disorganization and enhanced expression of smooth muscle differentiation proteins, including smooth muscle actin. These results demonstrate a requirement for endoglin in descendants of Pax3-expressing vascular cell precursors, and thus provides new insight into the cellular basis underlying adult vascular diseases such as HHT.
Keywords: HHT, endoglin, Pax3, intersomitic, vascular malformation, dorsal aorta, intersomitic
Introduction
The transforming growth factor beta (TGFβ) and bone morphogenetic protein (BMP) receptor pathways play critical but still incompletely understood roles in cardiovascular development, and represent key regulatory systems that govern normal vascular remodeling, the vascular response to injury, reperfusion following ischemic revascularization, and vessel maturation (David et al., 2008; Ricard et al., 2012). Autosomal dominant mutations in the TGFβ/BMP co-receptor endoglin (ENG) (McAllister et al., 1994), or the type-I receptor, activin-like kinase-1 (ACVRL1; also known as ALK1) (Johnson et al., 1996), cause the hereditary vascular diseases hereditary hemorrhagic telangiectasia (HHT) types 1 and 2 (HHT1, HHT2), respectively. These related disorders affect between 1 in 5000 to 8000 people in the US, though this may be an underestimate (Easey et al., 2003). HHT patients present with symptoms that include debilitating nose bleeds (epistaxis) (Sabba et al., 2007) that negatively impact their quality of life (Pasculli et al., 2004), and brain, lung, liver and intestinal arteriovenous malformations (AVM), which can result in mortality (Giordano et al., 2006; Kjeldsen et al., 2000). In HHT, the maturation phase of angiogenesis appears to be affected, as mice with endoglin- or Alk1-deficient vessels, and human telangiectases, show loss of integrity, signs of defective pericyte investment (Bailly et al., 2010; Braverman et al., 1990; Lebrin et al., 2010), and altered levels of vessel-associated monocytes (Bourdeau et al., 2000; Sanz-Rodriguez et al., 2004). A more complete understanding of the functions of endoglin during the processes of vascular development and vessel homeostasis is critical to developing improved therapies for HHT.
Endoglin null (Eng−/−) embryos die at embryonic day (E) 10.5-11.5 due to defects in cardiac morphogenesis and angiogenesis due, in part, to an absence of differentiated vascular smooth muscle cells (Arthur et al., 2000; Bourdeau et al., 1999; Li et al., 1999). We have previously shown that endoglin is required in non-endothelial neural crest stem cells for maintenance of myogenic potential (Mancini et al., 2007). Moreover, in endoglin null embryos, conditional expression of an endoglin transgene in either endothelial or smooth muscle cells partially rescues smooth muscle cell differentiation and investment of the developing E9.5-E10.5 dorsal aorta (Mancini et al., 2009). Thus, endoglin's functions during angiogenesis are complex, likely comprising both cell-autonomous (e.g., adhesive, TGFβ/BMP signaling), autocrine, and paracrine (e.g., regulation of chemokine and matrikine expression (Young et al., 2012; Young et al., 2015)) signaling roles in multiple cell types that contribute to embryonic blood vessel development. However, the requirement for endoglin expression in vascular cell precursors for vessel formation remains unclear.
Vascular smooth muscle cells originate from multiple cell types during embryogenesis, including cells migrating from the neural crest and the lateral plate mesoderm (Majesky, 2007). Dermomyotome cells of the somite, marked by Pax3 expression, give rise not only to smooth muscle cells of the dorsal aorta (Esner et al., 2006), but also to a subpopulation of endothelial cells in the aortic endothelium (Esner et al., 2006; Stoller et al., 2008) and intersomitic vessels (Mayeuf-Louchart et al., 2014). Therefore, to assess the cell-autonomous requirement of endoglin for the contribution of Pax3-positive vascular cell precursors to the embryonic dorsal aorta and intersomitic vessels, we examined the consequences of conditional deletion of endoglin in Pax3-derived cells.
Materials And Methods
Mice
The Engtm1Hma strain has a targeted mutation in the endoglin (Eng) gene (MGI:2177822) (Allinson et al., 2007; Arthur et al., 2000) and the Engtm2.1Hma strain has loxP sites flanking exons 5-6, generating a conditional allele activated by Cre recombinase (MGI:3712802) (Allinson et al., 2007; Arthur et al., 2000). Both Eng alleles were obtained from Helen Arthur (Institute of Genetic Medicine, Newcastle University, Newcastle upon Tyne, UK.). The Tg(Wnt1-cre)11Rth strain (MGI:2386570, (Danielian et al., 1998)) and the Pax3tm1(cre)Joe (MGI:3573783, (Lang et al., 2005)) were obtained from the Jackson Laboratory (Bar Harbor, ME). The Gt(ROSA)26Sortm1Sor strain contains a Cre-inducible lacZ gene, and was obtained from Jackson Laboratory (Soriano, 1999). All mice were maintained in the C57BL/6 background. Noon of the day of vaginal plug observation was designated E0.5. Breeding, maintenance, and experimentation were conducted according to the NIH standards established in the Guidelines for the Care and Use of Experimental Animals. All studies were approved by the Maine Medical Center Institutional Animal Care and Use and Institutional Biosafety Committees.
PCR genotyping
Primers used for polymerase chain reaction genotyping of parental mice and embryonic tissue were: Eng Flox, CCATTCTCATCCTGCATGGTCC and CCACGCCTTTGACCTTGCTTCC; Eng+/− allele beta galactosidase (LacZ), GTCGTTTTACAACGTCGTGACT and GATGGGCGCATCGTAACCGTGC; Eng+/− null allele, GAGCTCGGTCAGAGGTCAAAG and CTGGGACGTCGTATGGGTAG; Eng Flox recombination, GGTCAGCCAGTCTAGCCAAG and CCACGCCTTTGTCCTTGC; Cre allele, GCTGGTTAGCACCGCAGGTGTAGAG and CGCCATCTTGCAGCAGGCGCACC.
Analysis of mouse tissues
All mice and embryos were genotyped twice: after birth and following euthanasia, as described (Mancini et al., 2009; Romero et al., 2011). Mice were anesthetized with avertin, and embryos were whole mount stained for beta-galactosidase activity, processed for paraffin embedding, and sectioned, harvested, fixed, and stained for beta galactosidase with eosin counterstaining as described previously (Mancini et al., 2009).
Histology, Immunohistochemistry, and Intracardiac Ink Injections
Antibodies used for immunofluorescence and immunohistochemistry were: α-SMA-CY3 conjugated (Sigma), α-CD34 (Pharmingen). The slides were examined with a Zeiss Axioskop microscope (Thornwood, NY, USA). Imaging and color histogram analysis were performed using Image J software. Histological analysis, immunohistochemistry for PECAM-1 (CD31; BD Pharmingen) and intracardiac India ink injections were performed as described previously (Krebs et al., 2004; Krebs et al., 2000). Aortic sectional areas were measured on serial images using the FIJI version of ImageJ. Significance of area differences was determined using Student's T test on three measurements on three separate cells per stack element.
Confocal Microscopy
Control and mutated Pax3Cre/+;EngLoxP/− mouse embryos at E10.5 were formalin fixed, then clarified by stepwise immersion in glycerol at 25%, 50%, 75%, 90% and 100% v./v. Embryos were counterstained with mouse isolectin IB4 (Abcam) and rabbit antibody to PDGFRβ (AbCam), followed by Alexa 488 conjugated anti-mouse IgG antibodies and Alexa 546 anti-rabbit IgG antibodies. Immunofluorescently stained embryos were studied using a Leica SP8 confocal microscope with X10 objective. The stacks of optical sections covering the full depth of laterally positioned embryos were collected and 3D reconstruction was achieved using the IMARIS program.
RNA Extraction and Real Time Quantitative RT-PCR Analysis
RNA isolation and real-time quantitative RT-PCR (qRT-PCR) analyses were carried out essentially as described (Young et al., 2012). For these experiments, E10.5 embryos were washed in PBS. Embryos were cut just below the forelimbs and above the hindlimbs, and the resulting thoracic segment was used for total RNA isolation using RNeasy plus (Qiagen). qRTPCR reactions were run in triplicate on a BioRad iQ5 system. Primers used for quantitative RTPCR of mouse embryo cDNAs are listed below:
Endoglin, AGCCCCACAAGTCTTGCAG and GCTAGTGGTATATGTCACCTCGC; Smooth muscle alpha actin, AAATCAGACATGTGCTACCC and TCAAATACC CCGTTTACATC; Smooth muscle myosin heavy chain (SMMHC1), AGAGCAAACTCAGGAGAGGA and TCACTGGCTTTGGTTCCATT; SM22alpha, CCTTTAAACCCCTCACCCAG and CGTAGGATGGACCCTTGTTG; Smooth muscle calponin1 (CNN1), GCATGACAGTGTTGGGCTT and CTCACTCAGCTCTGGGTACT. Normalization primers for qRTPCR analysis were: Mouse β2-microglobulin, CTGACCGGCCTGTATGCTAT and CCGTTCTTCAGCATTTGGAT. Statistical significance is presented as the p value, with significance set to p=0.05 or less.
Results
We previously found that transgenic expression of endoglin in endothelial and SM22-alpha-positive Eng−/− mesodermal cells produced significant cell-autonomous effects on vascular smooth muscle cell differentiation in vivo (Mancini et al., 2009). Therefore, to investigate endoglin loss-of-function in neural crest-derived vascular smooth muscle cell precursors, we combined a conditional endoglin null allele (EngLoxP/LoxP (Allinson et al., 2007)) with Pax3Cre (Li et al., 2000). Unlike Wnt1Cre (Brault et al., 2001), Pax3 is expressed in paraxial mesoderm and somite derivatives (Engleka et al., 2005; Mansouri et al., 2001; Schubert et al., 2001) that previous work suggests (Esner et al., 2006) contributes to the vascular precursor cells forming the dorsal aorta. Therefore, endoglin excision was driven by Pax3Cre to determine whether its expression is required for vascular precursor cell contributions to endothelial and vascular smooth muscle cells comprising the mouse vasculature.
Pax3Cre-mediated deletion of endoglin is embryonic lethal
The conditional endoglin null allele (EngLoxP/LoxP) used here possesses LoxP sites that flank exons 5 and 6, permitting the creation of a null allele following recombination with Cre recombinase. To sensitize recombination at the endoglin locus, we first crossed Pax3Cre transgenic mice with Eng+/− mice (Li et al., 2000; Liu et al., 2006). Next, the resulting Pax3Cre;Eng+/− mice were crossed with homozygous EngLoxP/LoxP mice. No Pax3Cre; EngLoxP/− mice were born alive (Table 1). The Wnt1Cre strain (Brewer et al., 2004) was similarly used to target neural crest endoglin expression in mouse E10-11 embryos. However, no apparent phenotype was observed Wnt1Cre; EngLoxP/− mice (Table 1) and, therefore, this approach was not pursued further.
Table 1.
Progeny from Wnt1Cre;Eng+/− or Pax3Cre;Eng+/− crosses with EngLoxP/LoxP mice.
| Genotype | Positive/Total (%) |
|---|---|
| Tg(Wnt1-Cre);EngLoxP/+ | 21/63 (33.3%) |
| Eng LoxP/+ | 16/63 (25%) |
| Eng LoxP/− | 10/63 (15.9%) |
| Tg(Wnt1-Cre);EngLoxP/− | 16/63 (25.4%) |
| Pax3Cre/+;EngLoxP/+ | 13/40 (32.5%) |
| Eng LoxP/+ | 16/40 (40%) |
| Eng LoxP/− | 11/40 (27.5%) |
| Pax3Cre/+;EngLoxP/− | *0/40 (0%) |
Wnt1Cre;Eng+/− were crossed with EngLoxP/LoxP mice and 10 litters (63 mice) were analyzed.
Pax3Cre;Eng+/− were crossed with EngLoxP/LoxP mice and 9 litters (40 mice) were analyzed. If mice are viable, expected outcome is 25% of each of 4 genotypes.
Pax3Cre/+;EngLoxP/− vs. Tg(Wnt1-Cre);EngLoxP/−, P=0.005.
Pax3Cre;Rosa embryo staining indicates expression in vascular smooth muscle precursors and a subset of endothelial cells
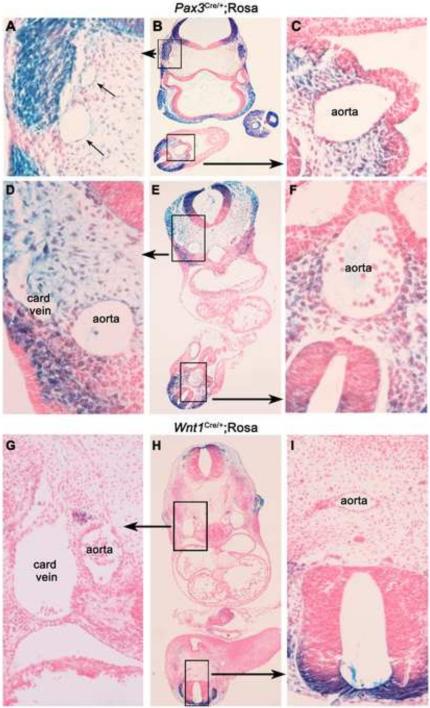
We visualized the distribution of recombination of the Pax3Cre and, for comparison, Wnt1Cre strains by examining the pattern of beta-galactosidase expression in a LacZ Cre reporter line (Soriano, 1999). As has been reported previously (Stoller et al., 2008), beta-galactosidase-positive (βgal+) cells derived from Pax3Cre-expressing cells were seen at E9.5 and E10.5 in the dorsal neural tube, throughout the neural crest, nearby mesenchyme, and to a lesser extent in the myotome (Fig. 1A-F). Also as reported (Stoller et al., 2008), we observed βgal-positive cells in the aorta (Fig. 1C), and also in vessels in the head region (Fig. 1A) and in the cardinal vein (Fig. 1D). βgal-positive cells were also found in some endothelial cells in the dorsal one third of the dorsal aorta at the heart level (Fig. 1D), but not at the caudal level (Fig. 1F). Therefore, detection of Pax3Cre activity in a subset of endothelial cells suggests that loss of endoglin expression in Pax3-derived rare endothelial cells can contribute to the Pax3Cre-mediated endoglin deletion phenotype. A similar analysis of the Wnt1Cre expression pattern showed strong βgal-positive cells in the dorsal neural tube and neural crest, but little evidence of expression in the developing vasculature (Fig. 1G-I).
Figure 1.
Recombination patterns of Pax3Cre and Wnt1Cre strains. Cre recombinase mouse strains were crossed to the Rosa Cre reporter strain (Soriano, 1999). Embryos were collected at E9.5 and E10.5, and whole mount stained for β-gal activity. Shown are E9.5 Pax3Cre/+;Rosa embryos at the level of the optic stalk and telencephalon (A-C) and at the level of the heart (D-F). (G-I) Wnt1Cre/+;Rosa sections were taken from an E10.5 embryo at the level of the heart. Boxed regions in middle column are expanded as indicated.
Conditional targeting Pax3-positive cells causes a vascular phenotype
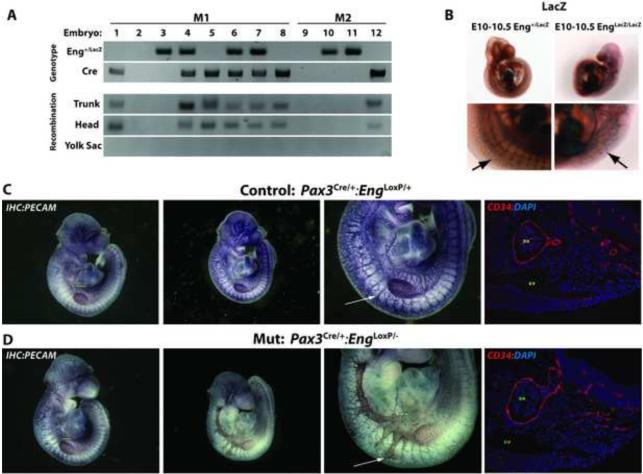
Pax3Cre;Eng+/− mice were used to generate tissue-restricted endoglin null (Pax3Cre;EngLoxP/−) mice. We confirmed Cre recombination of endoglin in embryos resulting from Pax3Cre/+;Eng+/− x EngLoxP/LoxP homozygote crosses. Embryos were positive for the EngLoxP allele (data not shown) and either or both of the Cre and LacZ-targeted Eng null alleles (Eng+/LacZ) (Arthur et al., 2000). Using published EngLoxP recombination-specific PCR primers (Allinson et al., 2007), we observed full correlation between the presence of the Pax3Cre allele and the Eng recombined allele in both head and trunk E9.5-E10.5 embryo sections (Fig. 2A, lanes 1, 4-7, and 12). Recombination was not observed in the embryonic yolk sac, consistent with a lack of Pax3-positive cell contribution to this tissue. Pax3Cre;EngLoxP/− embryos (lanes 4, 6, and 7) with presumptive abrogation of endoglin expression in Pax3-expressing cells presented as pale, with expanded and hemorrhagic vessels evident under light microscopy (data not shown). To confirm the systemic endoglin-null phenotype as previously described, Eng−/− embryos (at E10.5) were stained for LacZ expressed from the Eng null allele. These embryos bore all the features previously noted, including gross defects in vessel and heart development (Arthur et al., 2000; Bourdeau et al., 1999; Li et al., 1999). In particular, we also noted a profound defect in the formation of the intersomitic vasculature; the normally ordered reticulated intersomitic vasculature was highly disorganized in Eng−/− embryos, suggesting defective intersomitic vessel formation (Fig. 2B, arrows).
Figure 2.
The Pax3Cre allele efficiently recombines at the endoglin locus to generate a null allele and leads to abnormal intersomitic vessel morphology. Pax3Cre/+;Eng+/− mice were crossed with EngLoxP/LoxP homozygous mice. (A) Representative PCR was conducted on two litters from Pax3Cre/+;Eng+/− x EngLoxP/LoxP matings (M1, M2) of E10.5 embryos: Eng+/LacZ, the LacZ-targeted endoglin mutated allele; Cre, Cre recombinase transgene PCR product, the presence of which reflects the recombined null allele with exons 5 and 6 are deleted. (B) Examples of control or endoglin mutant (Eng−/−) E10.5 embryos stained for Eng locus-encoded LacZ beta-galactosidase activity. Arrows indicate Eng/LacZ expression in the control and Eng−/− intersomitic vessels. (C-D) Immunohistochemical endothelial cell-specific PECAM staining of control Pax3Cre/+;EngLoxP/+ and mutant Pax3Cre/+;EngLoxP/− embryos.. Arrows exemplify normal and malformed intersomitic vessels. DA, dorsal aorta; cv, cardinal vein. Immunofluorescence staining for CD34+ endothelial cells is shown in the panels to the right.
Endoglin targeting in Pax3-positive cells leads to intersomitic vessel misrouting
To visualize the vascular endothelium of Pax3Cre/+;EngLoxP/− mutant embryos and littermate controls at E10.5, we stained embryo whole mounts with a monoclonal antibody to platelet endothelial cell adhesion molecule-1 (PECAM-1), a vascular endothelial cell marker (Baldwin et al., 1994). As compared to littermate control embryos (Fig. 2C), Pax3Cre/+;EngLoxP/− embryos showed dilation of the intersomitic vessels, which appeared more advanced mid-embryo (Fig. 2D, panel 3, arrow). Immunofluorescence staining of sections in this region for CD34, which shows a specific endothelial cell distribution (Middleton et al., 2005), indicated a dilated vessel structure (Fig. 2D panel 4, arrow).
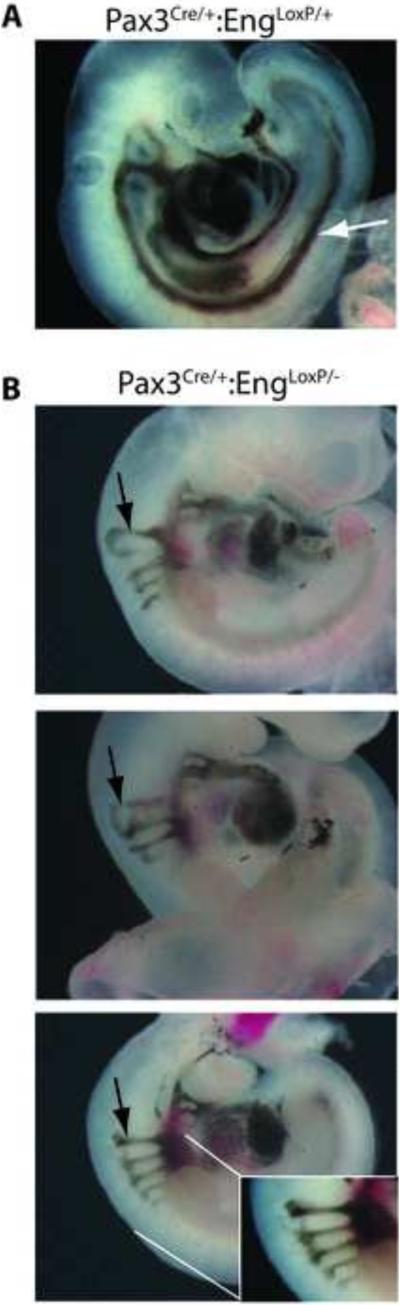
To assess vascular integrity, intracardiac India ink injections were performed as described (Krebs et al., 2010) on control and Pax3Cre;EngLoxP/− E10.5 embryos. In control littermate embryos (Fig. 3A), ink injected into the ventricle of the heart exited anteriorly through the paired branchial arch arteries, entered the paired dorsal aortae, and traversed caudally the entire length of the embryo. In Pax3Cre/+;EngLoxP/− embryos (Fig. 3B), injected ink exited through the paired branchial arch arteries, then aberrantly entered the intersomitic vessels (Fig. 3B), consistent with misrouting of the vessel. Caudally, the Pax3Cre/+;EngLoxP/− embryos exhibited an absence of ink/blood flow to caudal regions of the embryo. Whole mount staining of embryos using anti-smooth muscle actin antibody further revealed dilated aortae and cranial vessels (arrows, Fig. 4A, B, respectively).
Figure 3.
Pax3Cre-mediated endoglin recombination results in compromised intersomitic vessel integrity. Intracardiac India ink injections were performed on control Pax3Cre/+;EngLoxP/+ (A) and mutant Pax3Cre/+;EngLoxP/− (B) E10.5 embryos. Black arrows point to apparent misrouting of vessels corresponding to the junction of the cardinal vein- and aorta-derived intersomitic vessels. The white arrow (A) shows the flow of injected ink caudally through the dorsal aortae in the control embryo. Ink flow caudal to the heart is absent in the Pax3Cre/+;EngLoxP/− embryos (B).
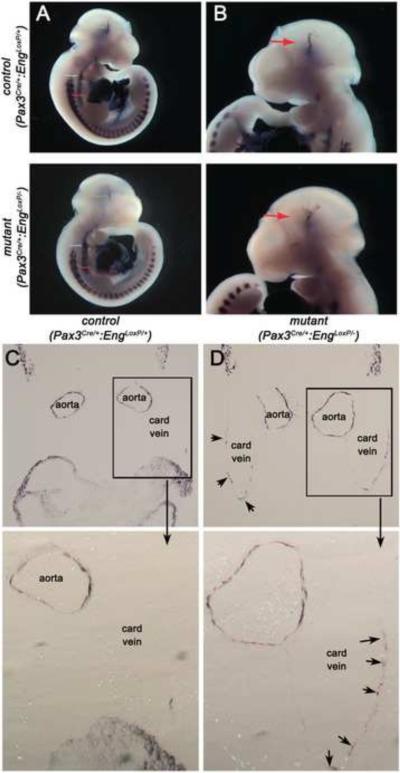
Figure 4.
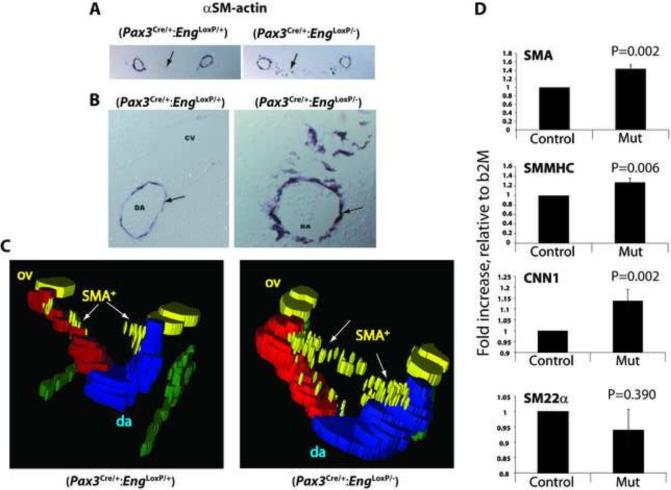
Loss of endoglin expression in Pax3Cre-expressing cells alters smooth muscle cell development and smooth muscle actin expression. (A, B) Whole mount vascular smooth muscle alpha actin (αSM-actin) staining of control Pax3Cre/+;EngLoxP/+ and mutant Pax3Cre/+;EngLoxP/− E10.5 embryos. Arrows point to (A) dilated dorsal aortae and (B) cranial vessels. (C, D) Sections showing αSM-actin positive cells in Pax3Cre/+;EngLoxP/+ (C) and mutant Pax3Cre/+;EngLoxP/− (D) E10.5 embryos. Distension of the paired aortae is apparent in the mutant, with ectopic SM-actin staining in the cardinal veins (arrows, card vein). Boxed areas are shown at higher magnification underneath.
Loss of endoglin in Pax3-positive cells leads to smooth muscle cell disorganization and enhanced smooth muscle actin expression
Profound aortic vessel dilation and disorganization of vascular smooth muscle cell layers was revealed by immunohistochemical smooth muscle actin staining of embryonic sections. Vessels exhibited larger diameters and reduced continuity between adjacent smooth muscle cells associated with the dorsal aortae (Fig. 4C). Potential sites of ectopic SM-actin were observed in the mutant cardinal vein (Fig 4D, arrows, and Fig. 7, below).
Figure 7.
Dorsal aortae exhibit level-specific altered smooth muscle marker expression. (A) αSM-actin-stained E10.5 control and mutant (B) embryo section that exhibits ectopic αSM-actin staining (area indicated by arrows). (B) Enlarged images showing disorganization of αSM-actin-positive cells adjacent to the dorsal aorta (Arrows). (C) Stack diagrams showing area measurements of aortic serial images. Colors in the reconstructions are: red and blue, the surfaces of the left and right branches of the paired dorsal aortae (da); green, the track of the dermal myotome; yellow, the otic vesicle (ov) and SMA-positive (SMA+) cells lying between the dorsal aortae (arrows). (D) qRT-PCR conducted on dissected embryo midsection corresponding to the region of ectopic αSM-actin expression. SMA, smooth muscle alpha actin; SMMHC, smooth muscle myosin heavy chain; CNN1, smooth muscle specific calponin-1; SM22α, smooth muscle specific transgelin.
Loss of endoglin in Pax3-positive cells results in intersomitic vessel malformation
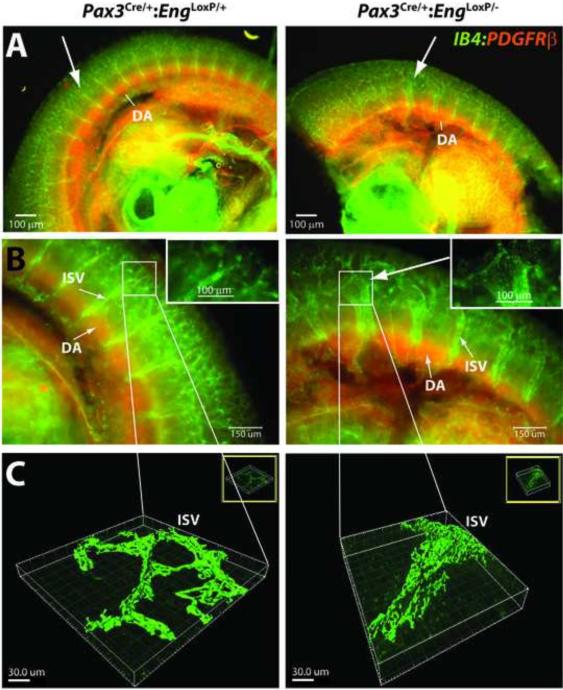
The midsection embryonic intersomitic vasculature was further examined using confocal microscopy. Embryos were whole mount stained with endothelial cell-specific Griffonia lectin and then counterstained with an antibody to pericyte-specific PDGFRβ protein. Confocal images revealed defects in the intersomitic vessels arising from the aorta (Fig. 5A). These defects presented as dilated arches (Fig. 5B, insets in expanded views) and three dimensional reconstruction of the dilations indicated inappropriate fusion and merging of the vessels (Fig. 5C).
Figure 5.
Pax3Cre/+;EngLoxP/- E10.5 embryos show intersomitic vessel malformation. (A) Whole mount fluorescent microscopic imaging of endothelial cell( Griffonia lectin IB4, green) and pericyte (anti-PDGFRβ, red) stained E10.5 embryos suggests profound intersomitic vessel malformation. DA, dorsal aorta. Arrows point to corresponding regions of normal (Pax3Cre/+;EngLoxP/+) and abnormal (Pax3Cre/+;EngLoxP/− ) intersomitic vessels. (B-C) Confocal microscopy (B) and three-dimensional reconstruction of the intersomitic vessels (C) showing abnormal organization of the intersomitic vessels. Scale bars are: Panels B, 150 μm; Panel B insets, 100 μm; Panels C, 30 μm.
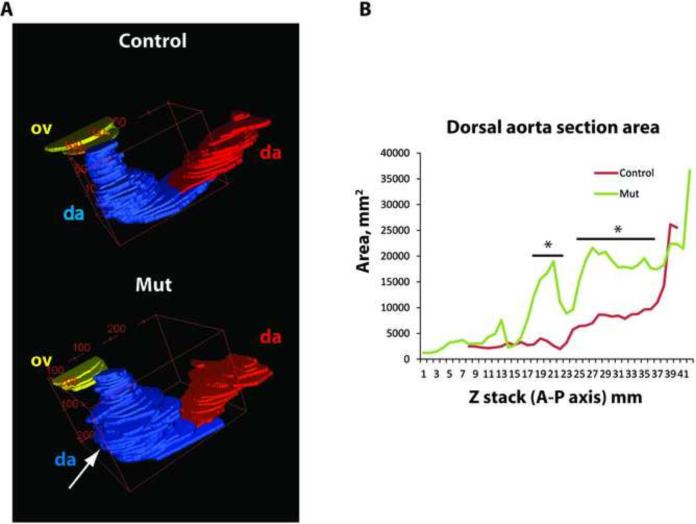
Pax3Cre-mediated loss of endoglin expression results in vessel dilation adjacent to the mid-embryo somites. To provide an estimate of the extent of anterior-posterior vessel dilation in mutant embryos, serial smooth muscle actin-stained vessel sections (as shown in Fig. 4B) were imaged throughout the embryo, the vessel luminal areas measured, and stacked. These measurements revealed variable enhanced dilations in the dorsal aortae (Fig. 6A), and confirmed that dilation was most extensive in the sections that corresponded to the mid-embryo somites (Fig. 6B). Similar serial section smooth muscle-stained reconstructions revealed the presence of significant ectopic smooth muscle actin-staining cells positioned between the paired dorsal aortae, but more anterior to the most dilated sections, in mutant embryos (Fig. 7A-C). In contrast to the global Eng-targeted mouse embryo (Bourdeau et al., 1999; Mancini et al., 2009), significant numbers of smooth muscle actin-positive cells are associated with the dorsal aorta, but appear less organized around the dilated mutant aorta (Fig. 7B). The location of the ectopic smooth muscle actin-staining cells was mid-level, as shown in three dimensional area reconstruction maps of serial smooth muscle actin-stained sections (Fig. 7C), which was consistent with data shown above (Figs. 2C and 2D).
Figure 6.
Dorsal aortae of Pax3Cre/+;EngLoxP/− E10.5 embryos are dilated. (A-B) ImageJ/FIJI morphometric analysis of serial sections of control and Pax3Cre/+;EngLoxP/− E10.5 aortae. Serial stacked area plates exhibit loss of uniformity (A) and increased area (B) indicative of dilatation. *, horizontal bars indicate regional area differences with p<0.05.
To assess possible alterations in vascular smooth muscle differentiation, cDNA was prepared from embryo thoracic sections and used for quantitative RT-PCR (qRT-PCR). These measurements confirmed the preceding results indicating that smooth muscle actin mRNA expression was elevated in the Pax3Cre/+;EngLoxP/− mutant embryos (Fig. 7D). Moreover, two additional late smooth muscle differentiation markers, smooth muscle myosin heavy chain and smooth muscle calponin1 were also significantly upregulated. This result contrasted with expression of SM22α, which was not significantly altered in the mutant as compared to control embryos (Fig. 7D).
Discussion
Angiogenesis is a multiple step process that involves recruitment of endothelial and smooth muscle cell precursors, and the intricate orchestration of signaling events within and between endothelial cells and vascular smooth muscle cells, which ultimately invest, stabilize, and promote the maturation of developing vessels. Pax3-positive cells mark somite-derived precursor cells that give rise to myogenic and vascular cell fates, the latter of which includes endothelial and vascular smooth muscle cell precursors. However, the identities of precursor cell populations and critical genes expressed therein that contribute to vessel formation and maturation remain to be elucidated.
The TGFβ/BMP coreceptor, endoglin, whose mutation causes one form of the vascular disease hereditary hemorrhagic telangiectasia (HHT, (McAllister et al., 1994)), is required for angiogenesis and vascular smooth muscle cell differentiation (Arthur et al., 2000; Bourdeau et al., 1999; Li et al., 1999), maintenance of myogenic potential in embryonic smooth muscle precursors (Mancini et al., 2007), and exhibits cell-autonomous roles for vessel development in both endothelial and vascular smooth muscle cells (Mancini et al., 2009). However, to date no conditional targeting of endoglin designed to define its requirement in a vascular cell precursor population during embryonic development has been performed. Therefore, we used Wnt1- and Pax3-based Cre recombinase-based reagents to explore the requirement for endoglin in vascular precursor cells.
Pax3Cre, but not Wnt1Cre-mediated deletion produced lethality in mice (Table 1). Although Wnt1 and Pax3 share some expression domains, the embryonic lineage of Pax3-expressing precursor cells is significantly broader by E10.5, including vascular structures. This is consistent with loss of endoglin in Pax3-derived cells leading to impaired vascular structure and embryonic lethality. This difference has also been observed previously. For example, systemic knockout of connexin 43 results in cardiac outflow tract defects with abnormal coronary vessel deployment. However, Wnt1Cre-mediated deletion did not recapitulate the connexin 43-null phenotype (Liu et al., 2006). In contrast, Pax3Cre deletion involves both ventral and dorsal aspects of the thoracic neural tube. Using Pax3Cre as the driver resulted in coronary anomalies similar to those seen in connexin 43-null hearts. Because Pax3 is expressed in rare endothelial cells and vascular smooth muscle precursors more broadly, the lethality of the Pax3Cre;EngLoxP/− phenotype indicated impairment of Pax3-positive precursors of both cell types.
Both the global endoglin null mouse and the ubiquitous conditional targeting of endoglin using PGK-Cre (Allinson et al., 2007) caused a similar E9.5-E10.5 lethal vascular phenotype. Our data contrast with studies of the global endoglin-targeted E10.5 mouse embryos, which appear to show a broader loss of vessel integrity (Li et al., 1999). In contrast to the global endoglin null E10.5 embryo, the most severe lesions appear at midlevel and smooth muscle actin-positive vascular smooth muscle cells are present adjacent to and investing the dorsal aorta. In addition, ectopic vascular smooth muscle cells expressing elevated levels of smooth muscle actin are seen more distant from the aorta. Defects in intersomitic vessel formation also appear most pronounced midsection, i.e., as a subset of the defects seen in the endoglin-targeted null mouse E10.5 embryo. This phenotype could be due to loss of endoglin expression in a subset of endothelial cells that were derived from Pax3-positive precursors (Esner et al., 2006; Stoller et al., 2008). This notion is supported by the observation that dilation of dorsal aorta and intersomitic arteries was mostly evident at the heart level but not the caudal region, such that the defects corresponded to the region of endothelial contribution of Pax3-positive derivatives.
As noted above, the smooth muscle actin-positive cells seen adjacent to vessels in the Pax3Cre;EngLoxP/− mutant contrasts with that seen in the global Eng−/− mutant (Arthur et al., 2000; Bourdeau et al., 1999; Li et al., 1999; Mancini et al., 2009). This observation suggests that other cells, possibly including Pax3-negative endothelial cells populating the aortic endothelium, can compensate for loss of endoglin in Pax3-positive precursor cells. Because Pax3-positive precursors contribute to both endothelial and smooth muscle constituents of the dorsal aorta, we cannot resolve whether the observed vessel dilatation or abnormal smooth muscle cell patterning is due to defective precursor cell autonomous effects in vascular smooth muscle cells, or endothelial cell-autonomous paracrine signaling defects that disrupt smooth muscle cell migration or organization. However, as the studies to date suggest that it is the endothelial role of endoglin that is critical for normal vessel formation (Tual-Chalot et al., 2015), it may be the endothelial cells derived from Pax3 positive vascular precursors responsible for the critical primary defect with associated disrupted smooth muscle organization as a secondary defect. Further work is required to dissect the relative contributions of Pax3 derived endoglin-deficient endothelial and smooth muscle cells to the vascular malformations.
PECAM whole mount immunostaining and intracardiac ink injections demonstrated that Pax3Cre;EngLoxP/− embryos exhibited malformation of the intersomitic vessel junctions and an absence of blood flow to caudal regions of the embryo. In a previous cardiac ink injection study of Eng−/− null embryos, dilation of the dorsal aortae and a similar absence of caudal blood flow were observed (Sorensen et al., 2003). However, the pattern of intersomitic vessel malformation observed in the present study was not evident in the prior study, suggesting that this phenotype is exacerbated in E10.5 Pax3Cre;EngLoxP/− embryos. The observed dilatation of the dorsal aorta and the intersomitic vessels seen in the present work is consistent with the vascular phenotype seen with Pax3-driven expression of the Notch1 intracellular domain (Mayeuf-Louchart et al., 2014), suggesting that activated Notch signaling in Pax3-positive precursors may suppress endoglin expression, which may contribute to elevation of TGFβ signaling resulting in elevated smooth muscle differentiation markers. Similar to our results, Notch pathway activation in Pax3-positive progenitors can result in an increase of the number of somatic smooth muscle and endothelial cells contributing to the aorta (Mayeuf-Louchart et al., 2014), which is consistent with these cells contributing to vascular smooth muscle regulation versus a myogenic fate. Further work will be required to evaluate this possibility.
The balance of Pax3 versus FoxC2 expression is a determinant of the myogenic- versus vascular fate of the respective cell precursors (Lagha et al., 2009). We were unable to detect changes in the levels of either Pax3 (adjusted for gene dosage due to the Cre allele) or FoxC1 by qRT-PCR (data not shown). However, any such changes in the targeted cells may have been masked by other cell contributions. Additionally, a recent study suggests that endoglin is regulated by Pax3 in the context of the endothelial cell and tumor progression (Fang et al., 2013). Because we did not see significant lethality or vascular defects (data not shown) for the Pax3Cre;Eng+/− genotype, we do not think Pax3 haploinsufficiency further reducing endoglin levels was sufficient to produce a significant phenotypic effect.
Endoglin and ALK1 are key mediators of endothelial cell-autonomous BMP9-dependent angiogenic signals (David et al., 2008; Ricard et al., 2012; Young et al., 2012). ALK1- and endoglin-dependent BMP9 targets in endothelial cells include stromal-derived factor-1 (SDF1) (Young et al., 2012) and monocyte chemoattractant protein-1 (MCP-1/CCL2) (Young et al., 2015). Altered expression of one or more of these factors due to endoglin deficiency can, therefore, potentially alter vessel structure or integrity. For example, the SDF1/CXCR4 is involved in coronary artery development (Ivins et al., 2015) and MCP-1 mediates TGFβ-dependent vascular smooth muscle cell migration (Ma et al., 2007). Thus, evaluation of these downstream endoglin-dependent BMP9 targets in vascular cell precursors may shed light on how deletion of endoglin may contribute to vessel fragility, malformation, and reduced vessel integrity and lead to new mechanistic insights into the cellular basis underlying HHT.
Highlights Young et. al.
First embryonic development study using conditional endoglin targeting, a target gene for the human vascular disease hereditary hemorrhagic telangiectasia.
Demonstration of an embryonic lethal phenotype with conditional deletion of endoglin in Pax3-positive cells.
Pax3Cre-driven deletion of endoglin causes disorganization of vascular smooth cell investment of the dorsal aortae.
Pax3Cre-driven deletion of endoglin causes intersomitic vessel malformation and leakiness
Loss of endoglin in Pax3-positive cells alters expression of smooth muscle differentiation genes.
Acknowledgements
This work was supported by the American Heart Association GRNT20460045 (CPHV), the National Institutes of Health National Center for Research Resources P20-RR-15555 (FR); NIH Grants HL083151 (CPHV) and 5R01HL10965 (LL), and Maine Medical Center. This paper is dedicated to the memory of Luke T. Krebs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allinson KR, Carvalho RL, van den Brink S, Mummery CL, Arthur HM. Generation of a floxed allele of the mouse endoglin gene. Genesis. 2007;45:391–395. doi: 10.1002/dvg.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, Burn J, Diamond AG. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- Bailly S, Dupuis-Girod S, Plauchu H. [Rendu-Osler disease: clinical and molecular update]. Med Sci (Paris) 2010;26:855–860. doi: 10.1051/medsci/20102610855. [DOI] [PubMed] [Google Scholar]
- Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- Bourdeau A, Cymerman U, Paquet ME, Meschino W, McKinnon WC, Guttmacher AE, Becker L, Letarte M. Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1. Am J Pathol. 2000;156:911–923. doi: 10.1016/S0002-9440(10)64960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest. 1999;104:1343–1351. doi: 10.1172/JCI8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Braverman IM, Keh A, Jacobson BS. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J Invest Dermatol. 1990;95:422–427. doi: 10.1111/1523-1747.ep12555569. [DOI] [PubMed] [Google Scholar]
- Brewer S, Feng W, Huang J, Sullivan S, Williams T. Wnt1-Cre-mediated deletion of AP-2alpha causes multiple neural crest-related defects. Dev Biol. 2004;267:135–152. doi: 10.1016/j.ydbio.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone Morphogenetic Protein-9 Is a Circulating Vascular Quiescence Factor. Circ Res. 2008;102:914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easey AJ, Wallace GM, Hughes JM, Jackson JE, Taylor WJ, Shovlin CL. Should asymptomatic patients with hereditary haemorrhagic telangiectasia (HHT) be screened for cerebral vascular malformations? Data from 22,061 years of HHT patient life. J Neurol Neurosurg Psychiatry. 2003;74:743–748. doi: 10.1136/jnnp.74.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleka KA, Gitler AD, Zhang M, Zhou DD, High FA, Epstein JA. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol. 2005;280:396–406. doi: 10.1016/j.ydbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Esner M, Meilhac SM, Relaix F, Nicolas JF, Cossu G, Buckingham ME. Smooth muscle of the dorsal aorta shares a common clonal origin with skeletal muscle of the myotome. Development. 2006;133:737–749. doi: 10.1242/dev.02226. [DOI] [PubMed] [Google Scholar]
- Fang WH, Ahmed M, Wang Q, Li HM, Kumar P, Kumar S. PAX3 promotes tumor progression via CD105 signaling. Microvasc Res. 2013;86:42–43. doi: 10.1016/j.mvr.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Giordano P, Nigro A, Del Vecchio GC, Sabba C, De Mattia D. HHT in childhood: screening for special patients. Curr Pharm Des. 2006;12:1221–1225. doi: 10.2174/138161206776361192. [DOI] [PubMed] [Google Scholar]
- Ivins S, Chappell J, Vernay B, Suntharalingham J, Martineau A, Mohun TJ, Scambler PJ. The CXCL12/CXCR4 Axis Plays a Critical Role in Coronary Artery Development. Dev Cell. 2015;33:455–468. doi: 10.1016/j.devcel.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- Kjeldsen AD, Vase P, Green A. [Hereditary hemorrhagic telangiectasia. A population- based study on prevalence and mortality among Danish HHT patients]. Ugeskr Laeger. 2000;162:3597–3601. [PubMed] [Google Scholar]
- Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Starling C, Chervonsky AV, Gridley T. Notch1 activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis. 2010;48:146–150. doi: 10.1002/dvg.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- Lagha M, Brunelli S, Messina G, Cumano A, Kume T, Relaix F, Buckingham ME. Pax3:Foxc2 reciprocal repression in the somite modulates muscular versus vascular cell fate choice in multipotent progenitors. Dev Cell. 2009;17:892–899. doi: 10.1016/j.devcel.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Lang D, Lu MM, Huang L, Engleka KA, Zhang M, Chu EY, Lipner S, Skoultchi A, Millar SE, Epstein JA. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- Lebrin F, Srun S, Raymond K, Martin S, van den Brink S, Freitas C, Breant C, Mathivet T, Larrivee B, Thomas JL, Arthur HM, Westermann CJ, Disch F, Mager JJ, Snijder RJ, Eichmann A, Mummery CL. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16:420–428. doi: 10.1038/nm.2131. [DOI] [PubMed] [Google Scholar]
- Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- Li J, Chen F, Epstein JA. Neural crest expression of Cre recombinase directed by the proximal Pax3 promoter in transgenic mice. Genesis. 2000;26:162–164. doi: 10.1002/(sici)1526-968x(200002)26:2<162::aid-gene21>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu F, Schneider AE, St Amand T, Epstein JA, Gutstein DE. Distinct cardiac malformations caused by absence of connexin 43 in the neural crest and in the non-crest neural tube. Development. 2006;133:2063–2073. doi: 10.1242/dev.02374. [DOI] [PubMed] [Google Scholar]
- Ma J, Wang Q, Fei T, Han JD, Chen YG. MCP-1 mediates TGF-beta-induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood. 2007;109:987–994. doi: 10.1182/blood-2006-07-036400. [DOI] [PubMed] [Google Scholar]
- Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Mancini ML, Terzic A, Conley BA, Oxburgh LH, Nicola T, Vary CP. Endoglin plays distinct roles in vascular smooth muscle cell recruitment and regulation of arteriovenous identity during angiogenesis. Dev Dyn. 2009;238:2479–2493. doi: 10.1002/dvdy.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini ML, Verdi JM, Conley BA, Nicola T, Spicer DB, Oxburgh LH, Vary CP. Endoglin is required for myogenic differentiation potential of neural crest stem cells. Dev Biol. 2007;308:520–533. doi: 10.1016/j.ydbio.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A, Pla P, Larue L, Gruss P. Pax3 acts cell autonomously in the neural tube and somites by controlling cell surface properties. Development. 2001;128:1995–2005. doi: 10.1242/dev.128.11.1995. [DOI] [PubMed] [Google Scholar]
- Mayeuf-Louchart A, Lagha M, Danckaert A, Rocancourt D, Relaix F, Vincent SD, Buckingham M. Notch regulation of myogenic versus endothelial fates of cells that migrate from the somite to the limb. Proc Natl Acad Sci U S A. 2014;111:8844–8849. doi: 10.1073/pnas.1407606111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, McCormick MK, Pericak-Vance MA, Heutink P, Oostra BA, Haitjema T, Westerman CJJ, Porteous ME, Guttmacher AE, Letarte M, Marchuk DA. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- Middleton J, Americh L, Gayon R, Julien D, Mansat M, Mansat P, Anract P, Cantagrel A, Cattan P, Reimund JM, Aguilar L, Amalric F, Girard JP. A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146. J Pathol. 2005;206:260–268. doi: 10.1002/path.1788. [DOI] [PubMed] [Google Scholar]
- Pasculli G, Resta F, Guastamacchia E, Di Gennaro L, Suppressa P, Sabba C. Health-related quality of life in a rare disease: hereditary hemorrhagic telangiectasia (HHT) or Rendu-Osler-Weber disease. Qual Life Res. 2004;13:1715–1723. doi: 10.1007/s11136-004-7865-y. [DOI] [PubMed] [Google Scholar]
- Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119:6162–6171. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D, O'Neill C, Terzic A, Contois L, Young K, Conley BA, Bergan RC, Brooks PC, Vary CPH. Endoglin regulates cancer-stromal cell interactions in prostate tumors. Cancer Res. 2011;71:3482–3493. doi: 10.1158/0008-5472.CAN-10-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabba C, Pasculli G, Lenato GM, Suppressa P, Lastella P, Memeo M, Dicuonzo F, Guant G. Hereditary hemorrhagic telangiectasia: clinical features in ENG and ALK1 mutation carriers. J Thromb Haemost. 2007;5:1149–1157. doi: 10.1111/j.1538-7836.2007.02531.x. [DOI] [PubMed] [Google Scholar]
- Sanz-Rodriguez F, Fernandez-L. A, Zarrabeitia R, Perez-Molino A, Ramírez JR, Coto E, Bernabeu C, Botella LM. Mutation Analysis in Spanish Patients with Hereditary Hemorrhagic Telangiectasia. Deficient Endoglin Upregulation in Activated Monocytes. Clinical Chemistry. 2004;50:2003–2011. doi: 10.1373/clinchem.2004.035287. [DOI] [PubMed] [Google Scholar]
- Schubert FR, Tremblay P, Mansouri A, Faisst AM, Kammandel B, Lumsden A, Gruss P, Dietrich S. Early mesodermal phenotypes in splotch suggest a role for Pax3 in the formation of epithelial somites. Dev Dyn. 2001;222:506–521. doi: 10.1002/dvdy.1211. [DOI] [PubMed] [Google Scholar]
- Sorensen LK, Brooke BS, Li DY, Urness LD. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Dev Biol. 2003;261:235–250. doi: 10.1016/s0012-1606(03)00158-1. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stoller JZ, Degenhardt KR, Huang L, Zhou DD, Lu MM, Epstein JA. Cre reporter mouse expressing a nuclear localized fusion of GFP and beta-galactosidase reveals new derivatives of Pax3-expressing precursors. Genesis. 2008;46:200–204. doi: 10.1002/dvg.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tual-Chalot S, Oh SP, Arthur HM. Mouse models of hereditary hemorrhagic telangiectasia: recent advances and future challenges. Frontiers in genetics. 2015;6:25. doi: 10.3389/fgene.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K, Conley B, Romero D, Tweedie E, O'Neill C, Pinz I, Brogan L, Lindner V, Liaw L, Vary CP. BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood. 2012;120:4263–4273. doi: 10.1182/blood-2012-07-440784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K, Tweedie E, Conley B, Ames J, FitzSimons M, Brooks P, Liaw L, Vary CP. BMP9 Crosstalk with the Hippo Pathway Regulates Endothelial Cell Matricellular and Chemokine Responses. PLoS One. 2015;10:e0122892. doi: 10.1371/journal.pone.0122892. [DOI] [PMC free article] [PubMed] [Google Scholar]