INTRODUCTION
Dendritic cells (DCs) play a key regulatory role in antitumor immunity, especially in its immune accessory role via MHC-I molecules[1-5]. We have recently reported that DCs were able to enhance the killing activity of Lymphokine and PHA activated killer (LPAK) cells in vitro[6-8]. In the present study, we evaluated the effects of GM-CSF and TNF upon antitumor activities of freshly isolated dendritic Cells in human peripheral blood (DC-0) and those cells cultivated for 36 h in vitro (DC-36). To perform such an evaluation, we compared killing effects of LPAK cells with addtional DC-0 or DC-36 on hepatoma cell line (BEL-7402) under regulation of GM-CSF or TNF. This study provided some basic data for further antitumor research.
MATERIALS AND METHODS
Tumor cell line
Human hepatoma cell line BEL-7402 was purchased from experimental center of Sun Yat-Sen University of Medical Sciences.
Isolation of DCs
According to our previous method[9], peripheral blood mononuclear cells (PBMNC) from healthy volunteers were prepared by using Ficoll-Hypaque (ρ = 1077 g/L) centrifugation method. Interface cells were collected and washed three times to remove platelets. Discontinuous Percoll density gradient centrifugation (Percoll: Pharmacia, Sweden) was employed, and then interface cells between 35% and 50% were collected which were called as preliminary enrichment of DCs and divided into two shares. One share (DC-0) was panned immediately; other one (DC-36) further cultured in PRMI-1640 with 100 mL/L inactivated fetal calf serum (100 mL/L FCS PRMI-1640) at 37 °C in a full humidified 50 mL/L CO2 atmosphere for 36 h, and panned. The non-adherent fractions of two shares as DC-0 and DC-36 were washed and collected for the experiments.
Preparation of LPAK cells[9]
The PBMNCs were prepared in the same procedure as above, cultured 2 × 109/L- population with the final concentration of rhIL-21000 ku/L and PHA 20 mg/L in 100 mL/L FCS PRMI-1640 at 37 °C in a full humidified 50 mL/L CO2 atmosphere for 7 d. Half volume of the solution was replaced by fresh culture medium at the fourth day.
Anti-tumor experiment
The anti-tumor experiments were divided into two groups and each contained five experimental subgroups. Two ratios of effect (LPAK) to target (BEL-7402) (5:1 and 10:1) were used in all groups. ① DC-0 group: d group: BEL-7402 (8 × 107/L) + LPAK + DC-0 (8 × 106/L); g1 group: d group + GM-CSF (500 ku/L); g2 group: d group + GM-CSF (100 ku/L); t1 group: d group + TNF (5000 ku/L); t2 group: d group + TNF (500 ku/L); ② DC-36 group: each experimental group was the same as that in DC-0 group except DC-36 in place of DC-0 in the same concentration. These experimental groups were called D group, G1 group, G2 group, T1 group and T2 group respectively. In addition, L groups as the corresponding control groups, BEL-7402 + LPAK, experimental control group only consisted of BEL-7402, its population was 8 × 107/L. Culture medium control group only contained 100 mL/L FCS-PRMI-1640 with the supernatant of LPAK cells at a concentration of 50 μL/culture well. All of these groups were cultured in 96-well-culture plates and each group had 3 wells at 37 °C in a full humidified 50 mL/L CO2 atmosphere for 48 h. Cytotoxity assay was detected by using neural red uptake method.
Cytotoxicity assay (neural red uptake method)[10]
0.1 mL 0.3 mL/L neural red solution was added in each well for another 1 h of culture. Following three washings with phosphate-buffered saline (PBS), 0.1 mL HCl-ethanol solution was added in each well. The absorption value (A value) of each well was immediately read by BIO-RAD 3550-UV type automatic ELISA reader at 570 nm wavelength. The formula of cytotoxicity is as follows: [1 - (Experimental group A - medium control group)/(Control group A - medium control group A)] × 100%. The experimental results were analyzed through analysis of variance by using GB-STAT statistic software. The experiment repeated four times at the same condition.
RESULTS
Influence of DC-0 and DC-36 on cytotoxity activity of LPAK cells
The cytotoxic activity of L group, d group and D group was enhanced when their ratios of effect to target increased (P < 0.01). Their cytotoxic activity were D group > d group > L group (P < 0.01) respectively while in the same ratio of effect to target (Figure 1).
Figure 1.
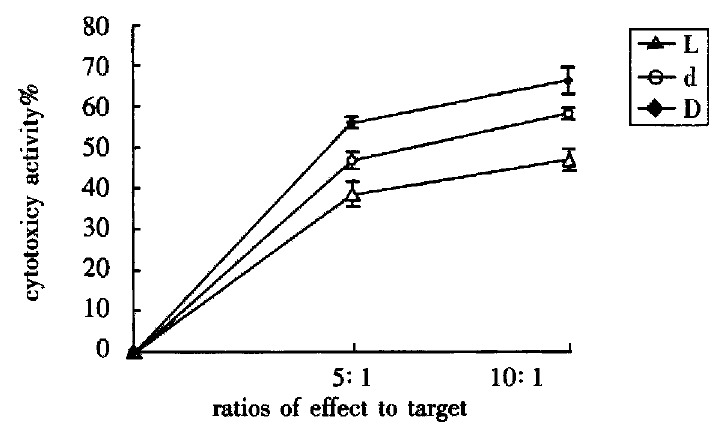
Influence of DC-0 and DC-36 on LPAK cells in killing BEL-7402 cells in vitro.
Influence of GM-CSF on DC-0 and DC-36 in helping LPAK cells killing effect
When there were two ratios of effect to target, cytotoxic activity of g1 group and g2 group were obviously higher than d group (P < 0.01), meantime, cytotoxic activity of G1 group and G2 group were greatly higher than D group (P < 0.01). However, the difference between g1 group and g2 group was not distinct (P > 0.05), also there were no difference between G1 group and G2 group (P > 0.05). But there were obviously different between g1 group and G1 group (P < 0.01), at the same time, the difference between g2 group and G2 group were distinct (P < 0.01) (Figure 2).
Figure 2.
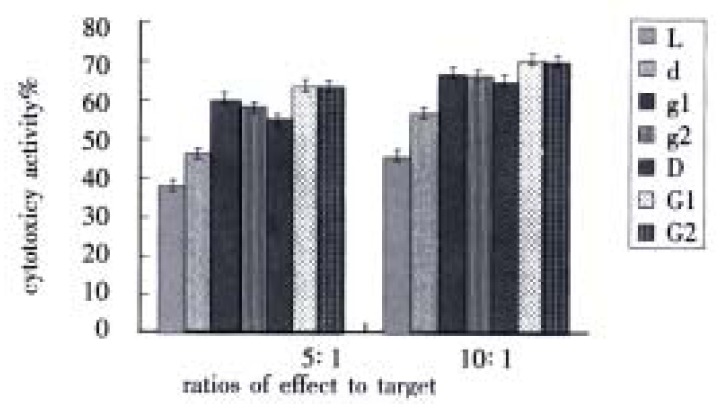
Influence of GM-CSF on DC-0 and DC-36 in helping LPAK cells killing activity in vitro.
Influence of TNF on DC-0 and DC-36 in helping LPAK cells killing effect
While there were two ratios of effect to target, cytotoxic activity of t1 group or t2 group was evidently higher than that of d group (P < 0.01), meantime, cytotoxic activity of T1 group or T2 group was markedly higher than that of D group (P < 0.01). However, the differences between t1 and t2 group, and between T1 and T2 group were distinct (P < 0.01). Furthermore, there were difference between t1 and T1 group (P < 0.01), at the same time, between t2 and T2 group (P < 0.01) (Figure 3).
Figure 3.
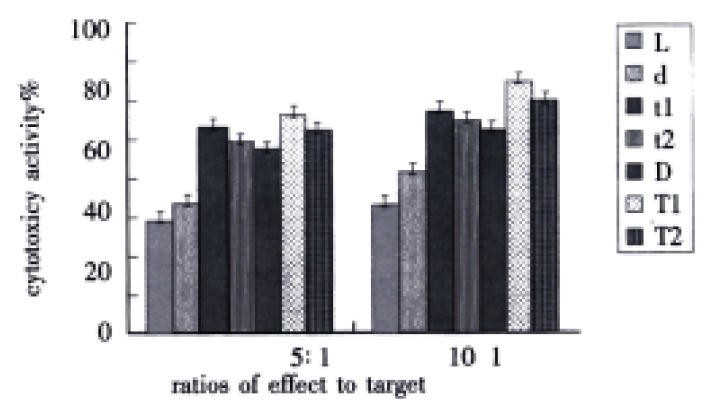
Influence of TNF on DC-0 and DC-36 in helping LPAK cells killing activity in vitro.
DISCUSSION
In recent years, it is considered that mature DCs in human peripheral blood have high stimulating function, which efficiently presents tumor-peptide epitopes leading to induce cytotoxic T lymphocytes (CTL) to produce stronger specific antitumor immune response[11-14]. LPAK cells after 7-d induction chiefly express similar phenotype with the CD16-, CD8+, CD3+ CTL subtype[15-18]. In our experiments, cytotoxic activity in D group was obviously higher than in d group, which demonstrated that the proportion of mature DCs in DC-36 group was higher than those in DC-0 group and DC-36 group could stimulate LPAK cells to exert stronger antitumor immune response. This effect suggested that a lot of precursor cells of DCs and immature DCs in freshly isolated DCs could differentiate into mature DCs after 36 h cultivation, which coincided with Young’s opinion[19]. In human peripheral blood, however, not all the precursor cells and immature DCs are able to automatically differentiate into mature DCs in vitro. It is GM-CSF that promotes the differentiation, maturation and activation of DCs. GM-CSF can not only initiate and promote development of DCs from MHC II-, MHC II+ precursors and immature DCs, but also upregulated CD86- expression on DCs, which make DCs to have activating and controlling antitumor immune function[20-24]. Cytotoxic activity difference between G2 (g2) group and G1 (g1) group were not distinct, this finding illustrated that maturation and activation of DCs did not result from one factor but from combination of multiple factors. In addition, the different time required in different developing stage of DCs populations must be considered. Although developing time in DC-36 group was longer than that in DC-0 group, not all DCs in DC-36 group differentiated into mature DCs. Therefore, increasing GM-CSF concentration alone was senseless. This phenomenon may be helpful in further study of antitumor immunity and clinical research.
In the case of TNF addition, cytotoxic activity was increased greatly, this finding attributed to three roles of TNF: 1, TNF is able to serve as the first signal, which affects DCs development in their early stage or whole stage, leading to upregulate the GM-CSF receptor level of DCs[21]. The supernatant of LPAK cells with minor quantity of cell growth factors such as TNF was added into culture medium to afford synergetic effect with GM-CSF; 2, TNF upregulates expression of CD80, CD83, CD86 and MHC-II in a short period[25,26]. Because these molecules are crucial for efficient antigen presenting, they promote the differentiation, development and activation of DCs. 3, TNF itself can kill tumor cells directly[27-32]. Compared with two t groups, cytotoxic activity of T1 and tª-1 groups were higher than that of T2 and t2 groups, which showed that in DC-36 groups there was plenty of time for immature DCs to evolve into mature DCs after the addition of TNF. Furthermore, T1 (t1) group had higher cytotoxic activity than T2 (t2) group, which was further increased when TNF dosage was raised. This phenomenon may attribute to antitumor effect of TNF itself and the synergetic effect between LPAK cells and TNF.
In conclusion, as compared with uncultured DC-0, cultured DC-36 from freshly isolated DCs had greater cooperative effect with GM-CSF or TNF. Moreover, they enable DCs to fulfill stronger antitumor effect.
Footnotes
Supported by Natural Science Foundation of the Higher Education Office of Guangdong Province, No. 19952901
Edited by You DY Proofread by Zhu LH and Ma JY
References
- 1.Girolomoni G, Ricciardi-Castagnoli P. Dendritic cells hold promise for immunotherapy. Immunol Today. 1997;18:102–104. doi: 10.1016/s0167-5699(97)01030-x. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Austyn JM. Dendritic cells. Curr Opin Hematol. 1998;5:3–15. doi: 10.1097/00062752-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Liu SC, Yuan SZ. Relationship between infiltration of dendritic cells, pericancerous lymphocytic reaction and prognosis in colorectal carclinomas. Xin Xiaohuabingxue Zazhi. 1997;5:156–157. [Google Scholar]
- 5.Hu JY, Wang S, Zhu JG, Zhou GH, Sun QB. Expression of B7 costimulation molecules by colorectal cancer cells reducestumorigenicity and induces anti-tumor immunity. World J Gastroenterol. 1999;5:147–151. doi: 10.3748/wjg.v5.i2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang JK, Chen HB, Sun JL, Zhou YQ. Effect of dendritic cells on LPAK cells induced at different times in killing hepatoma cells. Shijie Huaren Xiaohua Zazhi. 1999;7:673–675. [Google Scholar]
- 7.Sun JL, Zhang JK, Chen HB, Cheng JD, Qiu YQ. Promoting effects of dendritic cells on LPAK cells killing human hepatoma cells. Zhongguo Zhongliu Linchuang Yu Kangfu. 1998;5:16–18. [Google Scholar]
- 8.Chen HB, Zhang JK, Huang ZL, Sun JL, Zhou YQ. Effects of cytokines on dendritic cells against human hepatoma cell line. Shijie Huaren Xiaohua Zazhi. 1999;7:191–193. [Google Scholar]
- 9.Zhang JK, Chen HB, Wang J, Sun JL. Separating method of dendritic cells. Jiepou Kexue Jinzhan. 1998;4:272–273. [Google Scholar]
- 10.Zhu MS, Xu XY, Ding SB. Induction of LAK cells and detection of their activity. The basis of LAK cells and their clinic antitumor app lication. The first edition. Nanjing. Science & Technology Press of Jiangsu Province. 1993:48–67. [Google Scholar]
- 11.Li MS, Yuan AL, Zhang WD, Liu SD, Lu AM, Zhou DY. Dendritic cells in vitro induce efficient and special anti tumor immune response. Shijie Huaren Xiaohua Zazhi. 1999;7:161–163. [Google Scholar]
- 12.Li MS, Yuan AL, Zhang WD, Chen XQ, Tian XH, Piao YJ. Immune response induced by dendritic cells induce apoptosis and inhibit prolifera-tion of tumor cells. Shijie Huaren Xiaohua Zazhi. 2000;8:56–58. [Google Scholar]
- 13.Xiao LF, Luo LQ, Zou Y, Huang SL. Study of the phenotype of PBLs activated by CD28/.CD80 and CD2/.CD58 and acting with hepatoma cells and the restricted usage of TCR Vβ gene subfamily. Shijie Huaren Xiaohua Zazhi. 1999;7:1044–1046. [Google Scholar]
- 14.Zhai SH, Liu JB, Zhu P, Wang YH. CD54, CD80, CD86 and HLA ABC expressions in liver cirrhosis and hepatocarcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:292–295. [Google Scholar]
- 15.Huang SL, Xiao LF, Luo LQ, Chen HQ. Phenotype analysis and re-stricted usage of TCR Vβ genes subfamily in mAb costimulated T cells after incubated with hepatocellular carcinoma cell line. Huaren Xiaohua Zazhi. 1998;6:10 33–1035. [Google Scholar]
- 16.Tang ZY. Advances in clinical research of hepatocellular carcinoma in China. Huaren Xiaohua Zazhi. 1998;6:1013–1016. [Google Scholar]
- 17.Chen Q, Ye YB, Chen Z. Activation of killer cells with soluble gastric cancer antigen combined with anti-CD(3) McAb. World J Gastroenterol. 1999;5:179–180. doi: 10.3748/wjg.v5.i2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JK, Sun JL, Chen HB, Zhou YQ. Ultrastructural comparison of apoptosis of human hepatoma cells and LAK cells. Huaren Xiaohua Zazhi. 1998;6:877–879. [Google Scholar]
- 19.Young JW, Steinman RM. The hematopoietic development of dendritic cells: a distinct pathway for myeloid differentiation. Stem Cells. 1996;14:376–387. doi: 10.1002/stem.140376. [DOI] [PubMed] [Google Scholar]
- 20.Stingl G, Bergstresser PR. Dendritic cells: a major story unfolds. Immunol Today. 1995;16:330–333. doi: 10.1016/0167-5699(95)80148-0. [DOI] [PubMed] [Google Scholar]
- 21.Santiago-Schwarz F, Divaris N, Kay C, Carsons SE. Mechanisms of tumor necrosis factor-granulocyte-macrophage colony-stimulating factor-induced dendritic cell development. Blood. 1993;82:3019–3028. [PubMed] [Google Scholar]
- 22.Cao X, Zhang W, Wang J, Zhang M, Huang X, Hamada H, Chen W. Therapy of established tumour with a hybrid cellular vaccine generated by using granu-locyte macrophage colony stimulating factor genetically modified den-dritic cells. Immunology. 1999;97:616–625. doi: 10.1046/j.1365-2567.1999.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, Colombo MP. Dendritic cells infiltrating tumors cotransduced with granulocyte/mac-rophage colony stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med. 1999;190:125–133. doi: 10.1084/jem.190.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou QY, Li RB, Zheng PL, Yang LP, Chen YZ, Kong XP. Effect of embryo hepatic extracts on proliferation and differentiation of hepatoma BEL 7402 cells. Shijie Huaren Xiaohua Zazhi. 1999;7:243–245. [Google Scholar]
- 25.Morse MA, Zhou LJ, Tedder TF, Lyerly HK, Smith C. Generation of dendritic cells in vitro from peripheral blood mononuclear cells with granulocyte-macrophage-colony-stimulating factor, interleukin-4, and tumor necrosis factor-alpha for use in cancer immunotherapy. Ann Surg. 1997;226:6–16. doi: 10.1097/00000658-199707000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austyn JM. New insights into the mobilization and phagocytic activity of dendritic cells. J Exp Med. 1996;183:1287–1292. doi: 10.1084/jem.183.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang WJ, Huang ZY, Ding YQ, Zhang WD. Lovo cell line apoptosis induced by cycloheximide combined with TNFα. Shijie Huaren Xiaohua Zazhi. 1999;7:326–328. [Google Scholar]
- 28.Yan BG, Yang ZC, Huang YS, Liu ZY, Fu QF, He BB, Li A. Effect of delayed rapid fluid resuscitation on liver function in early stage postburn. Shijie Huaren Xiaohua Zazhi. 1999;7:573–575. [Google Scholar]
- 29.Wang YF, Wu XN, Wu Q, Zhang XQ, Chen XF, Zhou XH, Wen WQ, Chen WY. Biological significance of serum soluble tumor necrosis factor recep-tor I in hepatoma patients. China Natl J New Gastroenterol. 1996;2:89–91. [Google Scholar]
- 30.Zhang GQ, Yu H, Zhou XQ, Liao D, Xie Q, Wang B. TNF-α induced apoptosis and necrosis of mice hepatocytes. Shijie Huaren Xiaohua Zazhi. 2000;8:303–306. [Google Scholar]
- 31.Wang JY, Wang XL, Liu P. Detection of serum TNF-alpha,IFN-beta,IL-6 and IL-8 in patients with hepatitis B. World J Gastroenterol. 1999;5:38–40. doi: 10.3748/wjg.v5.i1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu MC. Progress in surgical treatment of primary hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:921–923. [Google Scholar]


