Figure 1. Putative differentiation lineage hierarchy of normal human breast.
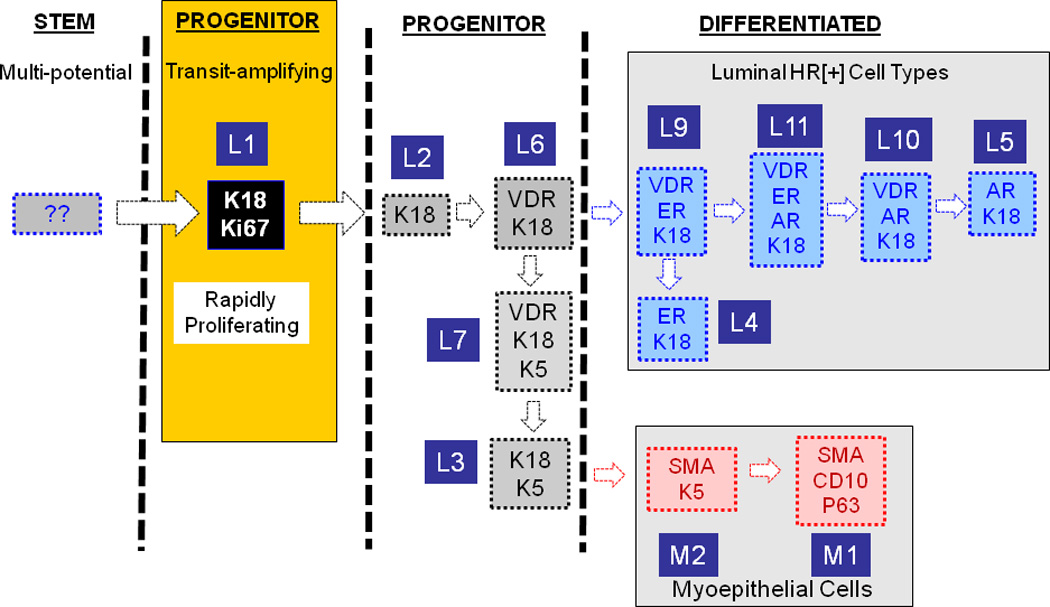
Stem cells are mostly quiescent and they rarely proliferate, but they give rise to a rapidly proliferating finite-lifespan progenitor cells with multi-lineage differentiation potential. The more differentiated cell types increasingly become mitotically less active, and finally the terminally differentiated cells become post-mitotic. Among the normal breast cell types only K18[+] cells were highly proliferative, which makes them the best candidate for the transit amplifying cells. We attempted to organize the rest of the breast cell types in a way where each differentiation step involves gain or loss of a single marker. Based on this constraint, we postulate that transit amplifying cell first loses its proliferative capacity which coincides with VDR expression giving rise to a oligo-potential progenitor (L6). This cell either maintains K18 expression and gains ER and AR expression giving rise to luminal HR+ cell types (L4–5, and L9–11), or it down-regulates K18 and up-regulate K5 (L7). When this cell downregulates VDR (L3) and upregulates SMA and p63 it generates the typical K5[+], HR[−] K18[−] myoeptihelial cell type (M2). As this cell downregulates K5 it generates the second subpopulation of K5[−] myoepithelial cells (M1).The above model depicts one possible scenario among many. Describing the interrelatedness of the breast cell types we identified and understanding their differentiation lineage hierarchy will require in vivo and functional ex vivo experiments that are not currently possible for technical reasons.
