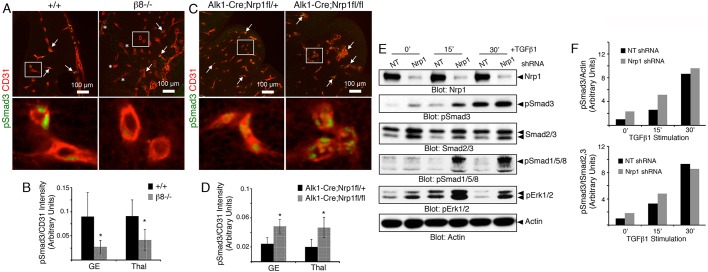
Fig. 6.
Nrp1 and β8 integrin cooperatively balance TGFβ signaling in brain endothelial cells. (A) Horizontal sections through the cerebral cortices of E12.5 wild-type and β8−/− embryonic brains were immunostained with anti-pSmad3 and anti-CD31 antibodies to visualize canonical TGFβ signaling in endothelial cells. Arrows indicate blood vessels containing nuclear pSmad3, whereas asterisks denote blood vessels lacking pSmad3. Lower panels are digitally magnified images of boxed areas in upper panels. (B) Quantitation of phosphorylated Smad3 levels in CD31+ endothelial cells within control and mutant cortical regions. Note the reduction in Smad3 phosphorylation in the β8−/− brain samples, *P<0.05, error bars represent s.d. (C) Horizontal brain sections from E13.5 Alk1-Cre control and Alk1-Cre;Nrp1fl/fl mutant embryos were immunostained with anti-pSmad3 and anti-CD31 to visualize TGFβ signaling in endothelial cells. Arrows indicate blood vessels containing nuclear pSmad3. Lower panels are higher magnification images of boxed areas in upper panels. (D) Quantitation of phosphorylated Smad3 levels in CD31+ endothelial cells within control and mutant cortical brain regions. Note that endothelial cells lacking Nrp1 contain significantly elevated levels of phosphorylated Smad3, *P<0.05, error bars represent s.d. (E) Endothelial cells infected with lentiviruses expressing GFP as well as non-targeting (NT) or Nrp1 shRNAs were stimulated with TGFβ1 for varying times and lysates were immunoblotted with the indicated antibodies. Note the higher levels of pSmad3, pSmad1/5/8 and pErk1/2 at baseline and following TGFβ1 stimulation. Nrp1-dependent differences in phosphorylated Akt1 or p38α were not detected. (F) Quantitation of Nrp1-dependent Smad3 phosphorylation levels before and after TGFβ1 stimulation based on the representative immunoblot in E, plotted as pSmad3 levels normalized to actin (upper graph) or normalized to total Smad2/3 (lower graph). GE, ganglionic eminences; Thal, thalamus.