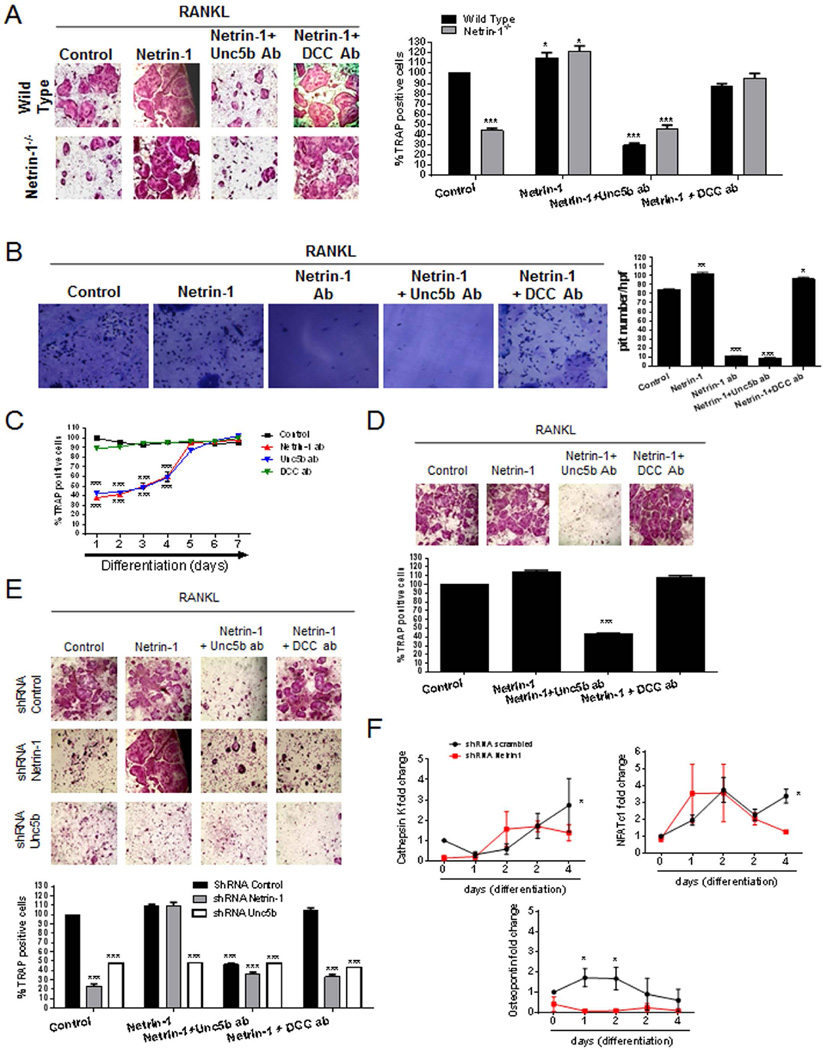
Fig. 3.
In vitro characterization of bone marrow–derived osteoclast. (A) WT and Netrin-1−/− mice osteoclast primary culture cells were fixed and stained for TRAP after being cultured for 7 days in the presence of recombinant Netrin-1 alone or combined with Unc5b or DCC antibodies. TRAP-positive cells containing three or more nuclei were counted as osteoclasts. (B) Toluidine blue staining to assay osteoclast activity. Nonadherent cells were seeded in dentin slides and treated for 7 days in the presence of recombinant Netrin-1 alone or combined with Unc5b or DCC antibodies. (C) Day response effect of Netrin-1 blockade on osteoclast differentiation. Netrin-1, Unc5b, and DCC antibodies were exposed to cultures at various time points after the start of the cultures. WT mouse osteoclast primary culture cells stained with TRAP to counteract osteoclast. (D) Human primary BMC-derived osteoclast culture cells were fixed and stained for TRAP after being cultured for 7 days in the presence of recombinant Netrin-1 alone or combined with Unc5b or DCC antibodies. TRAP-positive cells containing three or more nuclei were counted as osteoclasts. (E) RAW264.7 cells were permanently transfected with scrambled, Netrin-1, or Unc5b shRNA, and treated with 50 ng/mL RANKL together with recombinant Netrin-1 alone or in the presence of Unc5b and DCC antibodies. Cells were fixed and stained for TRAP after being cultured for 7 days. TRAP-positive cells containing three or more nuclei were counted as osteoclasts. (F) RAW264.7 cells were stably transduced with scrambled or Netrin-1 shRNA and treated with 50 ng/mL RANKL. Changes in cathepsin K, NFATc1, and osteopontin mRNA during the 4-day osteoclast differentiation process in Netrin-1 shRNA RAW264.7 cells were compared with scrambled shRNA-infected cells. All data are expressed as means ± SEM of four independent cultures. ***p < 0.001, *p < 0.05 related to control (ANOVA).