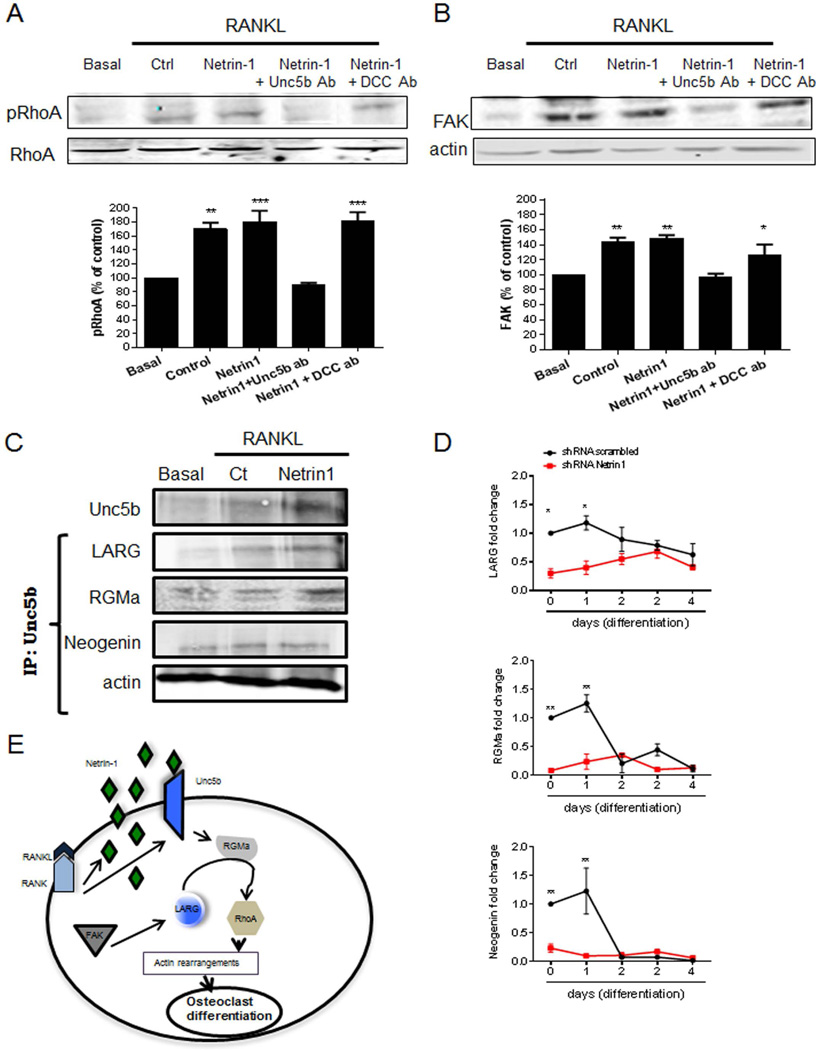
Fig. 7.
Netrin-1 and Unc5b interactions led to RhoA phosphorylation and FAK activation. Murine BMCs were treated with 30 ng/mL RANKL together with recombinant Netrin-1 alone or in combination with Unc5b or DCC antibodies. (A) RhoA phosphorylation was analyzed 15 minutes after stimulation by Western blot of lysates. (B) FAK expression was analyzed 15 minutes after stimulation by Western blot of lysates. To normalize for protein loading, the membranes were reprobed with RhoA or actin, respectively, and results normalized appropriately. (C) Cell extracts were immunoprecipitated with anti-Unc5b antibody. The immunoprecipitates were then analyzed by immunoblotting with anti-Neogenin, anti-LARG, and anti-RGMa antibodies. The figure shows representative data from one of four experiments. (D) RAW264.7 cells were stably transduced with scrambled or Netrin-1 shRNA and treated with 50 ng/mL RANKL. Changes in LARG, RGMa, and Neogenin mRNA during the 4-day osteoclast differentiation process in Netrin-1 shRNA RAW264.7 cells were compared with scrambled shRNA-infected cells. (E) Proposed intracellular pathway activated by Netrin-1/Unc5b to promote changes in RhoA cytoskeleton. The results were expressed as the means of four independent experiments. ***p < 0.001, **p < 0.01, *p < 0.5 versus nonstimulated control.