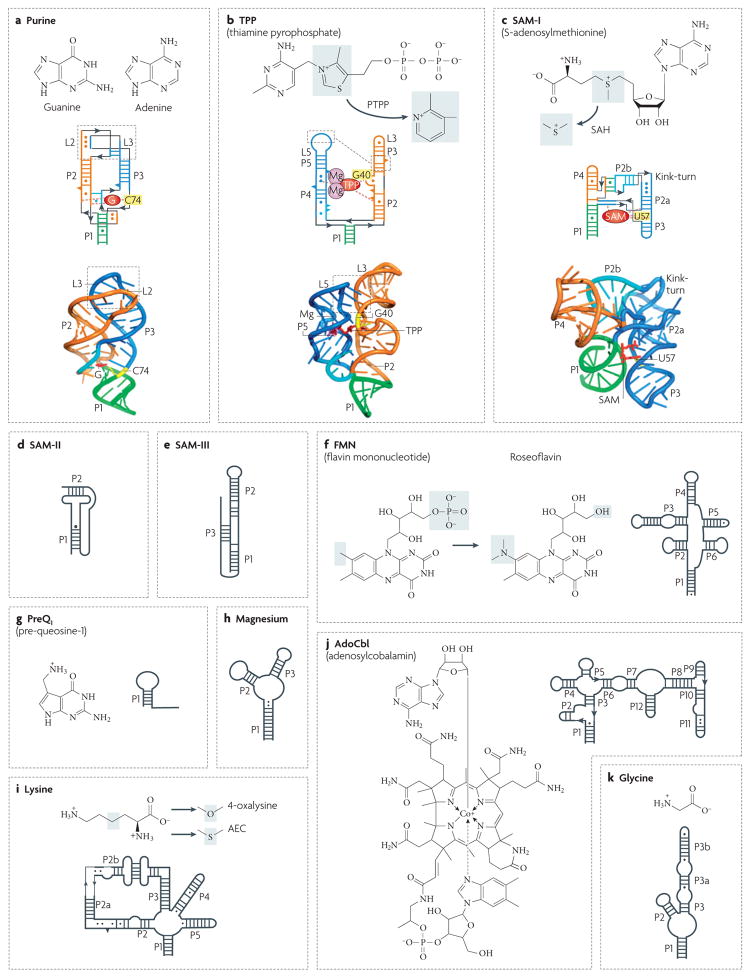
Figure 4. Secondary and tertiary structures of riboswitches.
Secondary structures are depicted by thick lines and are connected by black lines with arrows. Watson–Crick and non-canonical base pairs are shown as solid lines and circles, respectively. Natural riboswitch ligands and riboswitch-binding antibiotics are shown next to the secondary structures. Grey shadings indicate areas in which the ligands undergo changes. a | The Bacillus subtilis xpt gene guanine riboswitch bound to guanine (represented as a red G)66. The discriminatory nucleotide C74 is coloured yellow. b | The TPP riboswitch from the Escherichia coli thiM gene bound with TPP (in red)65. A pair of hydrated Mg2+ cations is shown in magenta. G40 interacting with the pyrimidine moiety of TPP is shown in yellow. The antibiotic pyrithiamine pyrophosphate (PTPP) differs from TPP by the central ring. c | The class I SAM Thermoanaerobacter tengcongensis riboswitch in complex with SAM (in red)64. U57 interacting with the purine moiety of SAM is shown in yellow. The grey area highlights a methyl group that is missing in S-adenosylhomocysteine (SAH). d | A class II SAM riboswitch from the Agrobacterium tumefaciens metA gene46. e | An SMK (class III SAM) riboswitch from the Streptococcus gordonii metK gene48. Helix P3 is formed by Shine–Dalgarno and anti-Shine–Dalgarno sequences. f | The FMN riboswitch from the B. subtilis ribD gene50. g | The preQ1 riboswitch from the B. subtilis queC gene56. h | The magnesium riboswitch from the Salmonella enterica mgtA gene57. i | The lysine riboswitch from the B. subtilis lysC gene54. j | The AdoCbl riboswitch from the E. coli btuB gene5. k | The glycine type II riboswitch from Vibrio cholerae gcvT gene53. Secondary structures in panels c, d, e, f, g, h, i, j and k modified with permission from Nature REF. 64 © (2006) Macmillan Publishers Ltd, REF. 46 © (2005) BioMed Central Ltd, Nature Structural & Molecular Biology REF. 48 © (2006) Macmillan Publishers Ltd, REF. 50 © (2002) National Academy of Sciences (USA), Nature Structural & Molecular Biology REF. 56 © (2007) Macmillan Publishers Ltd, REF. 57 © (2006) Cell Press, Nature Biotechnology REF. 61 © (2006) Macmillan Publishers Ltd, REF. 5 © (2002) Current Biology Ltd and REF. 53 © (2004) American Association for the Advancement of Science, respectively.