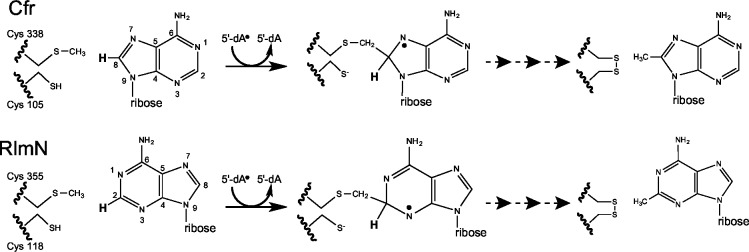
Fig 1. Mechanisms of action of Cfr and RlmN.
A simplified version of the mechanisms of action of Cfr and RlmN proposed by Grove et al [1, 2] starting with the methylated Cys 338/355 (as used in our computational approach) that has been generated by attacking the activated methyl group of the first SAM. Reductive cleavage of a second SAM gives an 5’-deoxyadenosyl 5’ radical, as shown in the figure, that abstracts a hydrogen atom from the mCys338/355 group to yield a neutral, carbon-centered radical. The resulting methylene radical adds to C8/C2 of A2503 in 23S rRNA generating a protein-RNA crosslink that contains an unpaired electron (not shown). Loss of an electron and abstraction of the proton (shown in bold) from C8/C2 by a general base, results in the resolution of the covalent crosslink by disulfide bond formation that involves a second cysteine (Cys105/118).